ADVERTISEMENT
Balloon Sizing of Pulmonary Branch Stenosis: A Useful Method to Guide Stent Implantation
ABSTRACT: Among patients with congenital heart disease, pulmonary branch stenosis is a common indication for stent implantation. Selective calibrated angiography is the standard method of vessel sizing to guide angioplasty balloon and stent selection. Our aim was to compare vessel dimensions from standard calibrated selective angiography with those obtained using a compliant sizing balloon catheter. Methods. 9 patients with 11 pulmonary branch stenoses underwent selective calibrated angiography. Amplatzer sizing balloon catheters positioned across the stenoses were inflated with dilute contrast agent. Digital angiograms were repeated in the same projections. Measurements from both methods were analyzed statistically. Minimum, maximum proximal and maximum distal vessel diameters were all significantly larger (p < 0.01) when measured by the sizing balloon method. Angioplasty balloons and stent diameters were chosen according to the sizing balloon measurements. In 7 of 8 stented lesions, larger angioplasty balloon diameters were selected for stent implantation than would have been chosen by standard angiography. Calibrated selective angiography may undersize vessel diameters. Use of a compliant sizing balloon appears to offer an accurate method to guide stent implantation in pulmonary branch stenosis.
J INVAS CARDIOL 2003;15:437–438
____________________________________________________
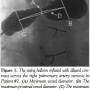 Intravascular stent implantation is the preferred treatment for native and post-operative branch pulmonary stenosis.1–4 The standard procedure for stent placement1 includes calibrated angiography, which has been regarded as the optimal calibration method for size estimation.5 The selection of angioplasty balloon catheter diameters and stents depends upon measurements of the stenosed vessel diameters determined by calibrated angiography. The aim of this technical report was to compare vessel dimensions determined by standard calibrated angiography to those obtained with the use of a compliant sizing balloon catheter. The impact of this comparison on the selection of the angioplasty balloon catheter diameter and stent was determined.
Intravascular stent implantation is the preferred treatment for native and post-operative branch pulmonary stenosis.1–4 The standard procedure for stent placement1 includes calibrated angiography, which has been regarded as the optimal calibration method for size estimation.5 The selection of angioplasty balloon catheter diameters and stents depends upon measurements of the stenosed vessel diameters determined by calibrated angiography. The aim of this technical report was to compare vessel dimensions determined by standard calibrated angiography to those obtained with the use of a compliant sizing balloon catheter. The impact of this comparison on the selection of the angioplasty balloon catheter diameter and stent was determined.
Methods
 After informed consent, nine consecutive patients (median age, 10 years; age range, 3–27 years; median weight, 37.7 kg; weight range, 14–106 kg) with 11 pulmonary branch stenoses were included in this study. Each patient underwent standard diagnostic catheterization and calibrated selective angiography of the affected pulmonary vessels. Subsequently, a super-stiff wire was positioned in the distal pulmonary artery and a 24 mm Amplatzer Sizing Balloon Catheter (AGA Medical Corporation, Golden Valley, MN) was passed over the wire until its midpoint was at the stenosis site. The balloon was then inflated with dilute (1:3) contrast agent and angiograms were obtained in the same projections as the calibrated angiograms. A marker pigtail catheter placed in the descending aorta and kept in the same position during both sets of angiograms was used for calibration and measurement of selected diameters from the images digitally stored on our catheterization lab digital system (Philips Integris BH 5000).
After informed consent, nine consecutive patients (median age, 10 years; age range, 3–27 years; median weight, 37.7 kg; weight range, 14–106 kg) with 11 pulmonary branch stenoses were included in this study. Each patient underwent standard diagnostic catheterization and calibrated selective angiography of the affected pulmonary vessels. Subsequently, a super-stiff wire was positioned in the distal pulmonary artery and a 24 mm Amplatzer Sizing Balloon Catheter (AGA Medical Corporation, Golden Valley, MN) was passed over the wire until its midpoint was at the stenosis site. The balloon was then inflated with dilute (1:3) contrast agent and angiograms were obtained in the same projections as the calibrated angiograms. A marker pigtail catheter placed in the descending aorta and kept in the same position during both sets of angiograms was used for calibration and measurement of selected diameters from the images digitally stored on our catheterization lab digital system (Philips Integris BH 5000).
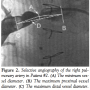
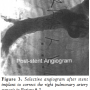 The following dimensions were measured in both the standard calibrated and the sizing balloon angiograms: minimum vessel diameter; maximum proximal vessel diameter; maximum distal vessel diameter; distance between the maximal proximal and maximal distal diameters. First, the minimum vessel diameter was determined on both angiograms. Then, the maximum proximal and maximum distal diameters were determined from the sizing balloon angiograms. The distances between the midpoint of the minimum diameter to the midpoints of both the maximum proximal and maximum distal diameters were measured from the sizing balloon angiograms. These distances were used to determine the vessel locations where maximum proximal and maximum distal diameters were measured on the calibrated angiograms. Calibration for measurements in both images was provided by the marker pigtail catheter positioned in the descending thoracic aorta. Figures 1 and 2 show the measurement technique studied in both the sizing balloon and the calibrated angiography methods. Figure 3 shows a selective angiogram after stent implantation. The two sets of measurements were then compared statistically using a paired t-test.
The following dimensions were measured in both the standard calibrated and the sizing balloon angiograms: minimum vessel diameter; maximum proximal vessel diameter; maximum distal vessel diameter; distance between the maximal proximal and maximal distal diameters. First, the minimum vessel diameter was determined on both angiograms. Then, the maximum proximal and maximum distal diameters were determined from the sizing balloon angiograms. The distances between the midpoint of the minimum diameter to the midpoints of both the maximum proximal and maximum distal diameters were measured from the sizing balloon angiograms. These distances were used to determine the vessel locations where maximum proximal and maximum distal diameters were measured on the calibrated angiograms. Calibration for measurements in both images was provided by the marker pigtail catheter positioned in the descending thoracic aorta. Figures 1 and 2 show the measurement technique studied in both the sizing balloon and the calibrated angiography methods. Figure 3 shows a selective angiogram after stent implantation. The two sets of measurements were then compared statistically using a paired t-test.
Results
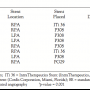
 The minimum vessel diameter, maximum proximal vessel diameter and maximum distal vessel diameter were larger when measured with the sizing balloon technique. The differences were statistically significant (p < 0.01). Angioplasty balloon and stent size were chosen in accordance with the sizing balloon measurements. Eight out of 11 stenotic lesions were successfully stented, two lesions had balloon dilation without stent implantation, and one lesion had mild stenosis and did not require intervention (Tables 1 and 2).
The minimum vessel diameter, maximum proximal vessel diameter and maximum distal vessel diameter were larger when measured with the sizing balloon technique. The differences were statistically significant (p < 0.01). Angioplasty balloon and stent size were chosen in accordance with the sizing balloon measurements. Eight out of 11 stenotic lesions were successfully stented, two lesions had balloon dilation without stent implantation, and one lesion had mild stenosis and did not require intervention (Tables 1 and 2).
Discussions
 This study compared pulmonary branch dimensions determined by calibrated standard angiography with those obtained by the use of a compliant sizing balloon catheter method. Dimensions determined by the compliant sizing balloon catheter method were significantly larger.
This study compared pulmonary branch dimensions determined by calibrated standard angiography with those obtained by the use of a compliant sizing balloon catheter method. Dimensions determined by the compliant sizing balloon catheter method were significantly larger.
In lesions having stent implantation, stents of the Palmaz 8 mm diameter series (Cordis Corp., Miami Lakes, FL) or the equivalent were selected. These stents allow dilation (or eventual redilation) to appropriate adult vessel diameters, and would have been the same using either sizing method. Angioplasty balloon catheters used for stent implantation were selected based on the diameters determined by the compliant balloon sizing method. Stent implantation was performed with angioplasty balloon catheter diameters equal to or slightly larger than the maximum distal diameter.
Because of differences in vessel diameters between the 2 sizing methods, larger implantation balloon diameters were selected using the compliant balloon sizing method than would have been selected using calibrated angiography. In 7 out of 8 lesions, larger balloon diameter catheters were selected (Table 2).
The significance of selecting larger balloon catheters in these cases may be two-fold. First, there may be less likelihood of stent slippage, malposition and/or embolization. Second, because of the larger implant diameters, there may be less need for stent redilation. A larger study would clearly be needed to demonstrate these advantages. In addition, the balloon sizing technique does not require injection of angiographic contrast into the patient’s circulation, thus decreasing the total patient contrast load. Similarly, the procedure can be repeated to acquire alternative views for accurate measurement of the vessel diameters or to study multiple stenotic lesions with different locations and configurations without the need for repeat contrast injection.
In conclusion, the use of a compliant sizing balloon appears to be an accurate method to guide implantation of stents to correct pulmonary branch stenosis. It may have important advantages over the use of standard calibrated selective angiography.
References
- O’Laughlin MP, Slack MC, Grifka RG, et al. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation 1993;88:605–614.
- Fogelman R, Nykanen D, Smallhorn JF, et al. Endovascular stents in the pulmonary circulation: Clinical impact on management and medium-term follow-up. Circulation 1995;92:881–885.
- Rosales AM, Lock JE, Perry SB, Geggel RL. Interventional catheterization management of perioperative peripheral pulmonary stenosis: Balloon angioplasty or endovascular stenting. Cathet Cardiovasc Intervent 2002;56:272–277.
- Formigari R, Santoro G, Guccione P, et al. Treatment of pulmonary artery stenosis after arterial switch operation: Stent implantation vs. balloon angiography. Cathet Cardiovasc Intervent 2000;50:207–211.
- Szmuilowicz E, Sklansky M, Kiciman N, et al. Evaluation of calibration methods for size estimation in the pediatric cardiac catheterization laboratory. Am J Cardiol 2000;83:313–318.
____________________________________________________
From the Heart Center for Children, St. Christopher’s Hospital for Children, Drexel University College of Medicine, Philadelphia, Pennsylvania.
Manuscript received February 14, 2003, and accepted March 25, 2003.
Address reprint requests to: John Moore, MD, Mattel Children’s Hospital at UCLA, 108333 Le Conte Ave., B2-427 MDCC, Los Angeles, CA 90095-1743.











