Association Between Primary Coronary Slow-Flow Phenomenon and Epicardial Fat Tissue
Abstract
Background. Primary coronary slow-flow phenomenon (CSFP) is defined as delayed opacification of contrast media in at least 1 coronary vessel in the absence of obstructive epicardial coronary artery disease (CAD) during coronary angiography. Epicardial fat tissue (EFT) surrounding coronary vessels provides paracrine effects. Released cytokines diffusing in the vessel wall may induce local inflammatory reactions that potentially result in endothelial dysfunction. The latter is thought to be the underlying cause of primary CSFP. However, to date, there are no data describing an association between EFT and CSFP. Therefore, the aim of the present study was to compare EFT thickness, clinical parameters, and outcomes in patients with and without CSFP. Methods. Coronary angiograms with primary CSFP obtained during a 10-year period were included in the analysis. EFT was measured in the 2-dimensional echocardiographic records. Clinical and diagnostic data were compared with non-CSFP patients who were matched for age, sex, and body mass index. Long-term follow-up was conducted by telephone interview. Results. A total of 48 CSFP patients (90% male; mean age, 64 ± 11.4 years) were identified, resulting in a prevalence of 0.13%. CSFP was observed in 87.5% in the left anterior descending artery, 50% in the right coronary artery, and 20.8% in the circumflex artery. Almost half of all patients showed CSFP in >1 vessel. There were no differences in baseline characteristics between CSFP patients and matched controls except for smoking history (31% vs 13%; P=.03). Median EFT thickness was significantly different between patients with and without CSFP (4.9 mm [interquartile range, 4.0-6.1 mm] vs 3.9 mm [interquartile range, 3.1-4.9 mm], respectively; P<.01). No differences in outcomes were observed. Conclusion. EFT is thicker in CSFP patients than in matched controls, but this appears to have no impact on long-term outcomes. Further studies are needed to elucidate the role of EFT in CSFP.
J INVASIVE CARDIOL 2021;33(1):E59-E64. doi:10.25270/jic/20.00294
Key words: coronary artery disease, coronary slow-flow, fat tissue
Coronary slow-flow phenomenon (CSFP) during coronary angiography is defined as delayed opacification in at least 1 coronary artery in the absence of significant obstructive epicardial coronary artery disease (CAD).1 The prevalence ranges between 1%-5.5%, depending on which criteria are used for the diagnosis. Patients with CSFP present primarily with recurrent chest pain that is often not associated with physical exertion and that frequently leads to repeated hospitalization.1,2 However, since its first description in 1972 by Tambe et al,4 the underlying pathophysiology still remains poorly understood, with “small vessel disease” or “microvascular dysfunction” being the most common terms in that context based on histopathological studies.1,3
Epicardial fat tissue (EFT) is located between the myocardium and the visceral pericardial layer directly surrounding the epicardial vessels and the myocardium without any borders or fascias.5,6 EFT has several protective functions, including mechanical protection of the epicardial vessels, supply of fatty acids to the myocardium in high demand states, and thermogenic effects.7 Conversely, as a metabolically active tissue, it is presumed to have paracrine effects, with cytokines like interleukin (IL)-6, IL-1ß, and tumor necrosis factor-alpha (TNF-α) diffusing and accumulating into the epicardial vessel wall and possibly inducing a local inflammatory reaction. This could potentially result in endothelial dysfunction, leading to increase of microvascular resistance7-10 and subsequently deteriorating coronary flow.
Our hypothesis is that EFT thickness is associated with CSFP. A correlation between EFT and the occurrence of CSFP has never been investigated. The aim of this study was to examine EFT in patients with and without CSFP. Furthermore, demographic, angiographic, and clinical characteristics were compared between the 2 cohorts.
Methods
Design and study population. A search was performed of all records of coronary angiograms (n = 36,923) obtained between January 2008 and December 2018 at the Kerckhoff Heart and Thorax Center in Bad Nauheim, Germany. Repeated examinations of the same patient during the study period were excluded. Records of 317 patients containing the terms “coronary slow-flow,” “delayed opacification,” and “delayed flow” were extracted. Patients with described slow-flow due to interventional treatment of coronary stenosis or occlusions, including patients presenting with acute coronary syndrome and patients with moderate and severe impaired myocardial left ventricular function as well as impaired flow due to coronary ectasia or aneurysms, were excluded. The remaining coronary angiograms were reviewed for CSFP by 2 experienced interventional cardiologists, resulting in a total of 48 CSFP patients. Echocardiographically measured EFT and clinical parameters of these patients were compared with a control cohort consisting of patients without CAD and CSFP. Patient cohorts were matched in terms of age, sex, and body mass index (BMI). Follow-up data were obtained by telephone interview or during repeat hospitalization after the index event. The primary endpoint was all-cause mortality and the secondary endpoint was acute myocardial infarction.
The study was approved by the local ethics committee of the University of Giessen, Germany (AZ 274/19).
Diagnostic criteria of CSFP. Based on studies by Beltrame et al,11 CSFP is defined as spontaneous delayed opacification of at least 1 epicardial vessel resulting in 3 or more beats for the dye to reach the distal end of the epicardial vasculature. Furthermore, coronary arteries should be normal or nearly normal, with a stenosis grade of no more than 40% by angiographic visualization. Exclusion criteria include slow-flow due to coronary emboli (eg, air, thrombus), coronary ectasia, and no-reflow phenomenon, as well as administration of exogenous vasoconstrictors (eg, cocaine).1,11
Measurement of EFT. EFT measurements were applied according to Iacobellis et al.12 Briefly, in 2-dimensional (2D) echocardiographic imaging, measurements were carried out separately in parasternal long-axis (PLAX) and parasternal short-axis (PSAX) views perpendicular to the right ventricular free wall, with the aortic anulus (PLAX) and the interventricular septum (PSAX) as landmarks. To avoid false measurements, careful attention was paid to distinguish between epicardial and pericardial fat layers. Whenever possible, measurements were made for a total of 3 cardiac cycles during end systole with calculation of the average value. Measurements were performed by 2 experienced echocardiographers.
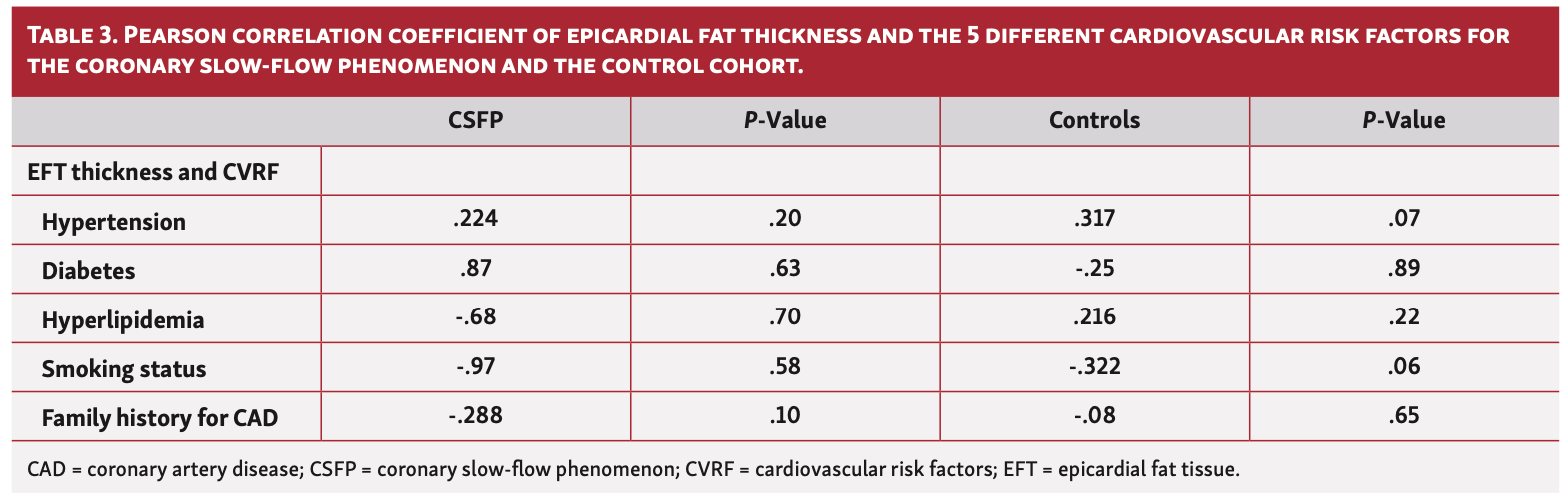
Statistical analysis. Continuous variables are presented as mean values ± standard deviations or as median values with interquartile ranges (IQRs), as appropriate. Categorical variables are reported as absolute values and percentages. A 2-sided Chi-square test was used to compare the 2 groups in terms of distribution of nominal variables. The Mann-Whitney U-test was used to compare metric parameters. The Kruskal-Wallis test was applied for comparison of variables between more than 2 groups. Pearson correlation was used for analysis of correlation between EFT thickness and BMI and EFT thickness and age as well as for EFT thickness and cardiovascular risk factors, and 1:1 matching was performed to identify patient populations with similar baseline characteristics in the CSFP and control cohorts. The following parameters were included: age, gender, and BMI. All statistical tests were conducted with a level of significance of <.05. SPSS, version 22.0 (IBM) was used for all statistical analyses.
Results
Baseline characteristics of the CSFP cohort. A total of 48 patients were diagnosed with CSFP, which corresponds to a prevalence of 0.13%. The mean age was 64 ± 11.4 years, and almost 90% were men. The mean BMI was 29.2 ± 4.6 kg/m2. The majority of CFSP patients (72.9%) had arterial hypertension. Hyperlipidemia was present in nearly 40%, there was a history of nicotine use in 31.3%, a positive family history for CAD was present in 16.7%, while diabetes was present in 8.3%. Since relevant (>40%) coronary artery stenosis was an exclusion criterion, only 2 patients in the cohort had a history of 1-vessel disease with prior PCI in a vessel not affected by CSFP. Two-thirds had coronary artery sclerosis whereas one-third were without CAD. No significant differences in cardiovascular risk factors were observed between the CSFP and control groups except for nicotine use (Table 1). CSFP patients suffered more frequently from symptoms of angina pectoris compared with the control group (62.5% vs 29.2%, respectively; P<.01). There was no difference in positive non-invasive ischemia testing between the groups (20.8% for the control cohort vs 22.9% for CSFP group; P=.80).
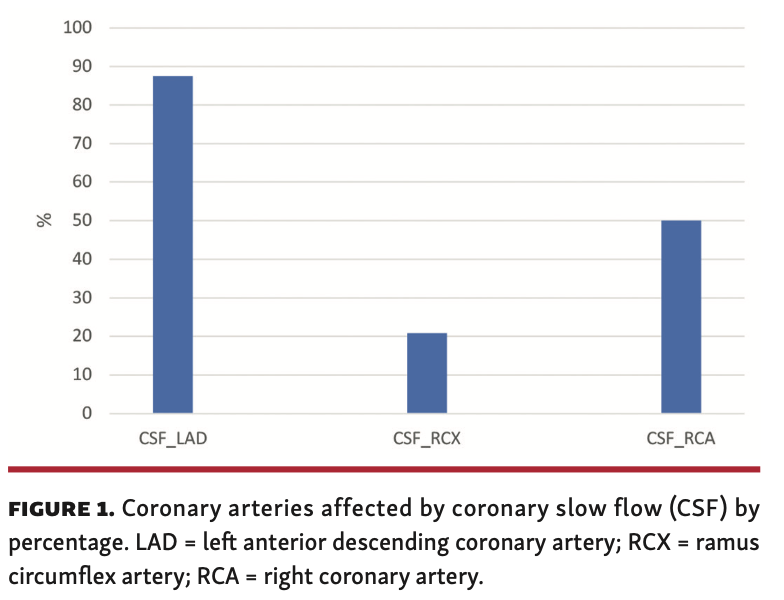
Coronary slow-flow vessel involvement. CSFP occurred most frequently (87.5% of all cases) in the left anterior descending (LAD) coronary artery. The right coronary artery (RCA) was affected in 50% of all patients and the circumflex artery was affected in 20.8%. Three-vessel involvement was present in 6 patients (12.5%). Almost half of all patients (46%) had CSFP in >1 vessel. When >1 vessel was involved, LAD and RCA was the most frequent combination (29.2%). Figure 1 shows the coronary distribution of CSFP. The mean BMI was 29.1 ± 4.4 kg/m2 when only 1 vessel was affected by CSFP, 28.7 ± 4.9 kg/m2 for 2-vessel involvement, and 31.1 ± 5.2 kg/m2 for 3-vessel involvement; there was no significant difference between these values (P=.45).
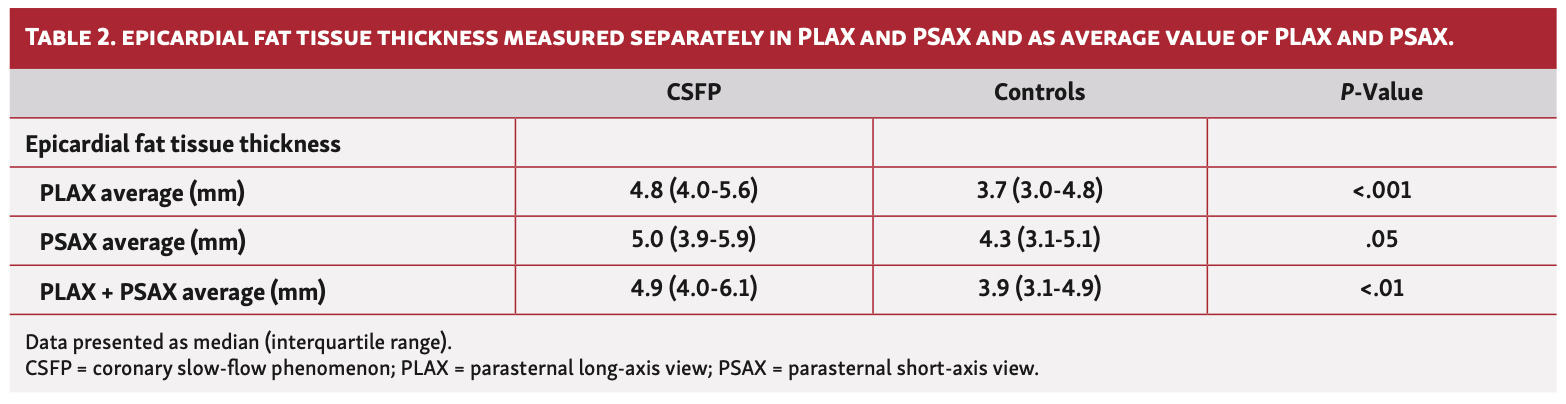
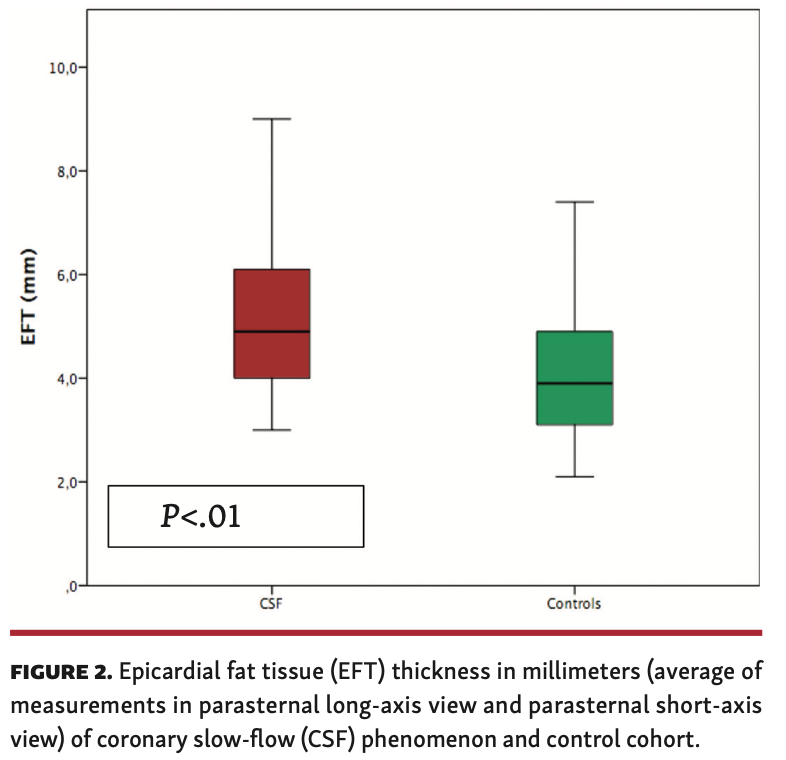
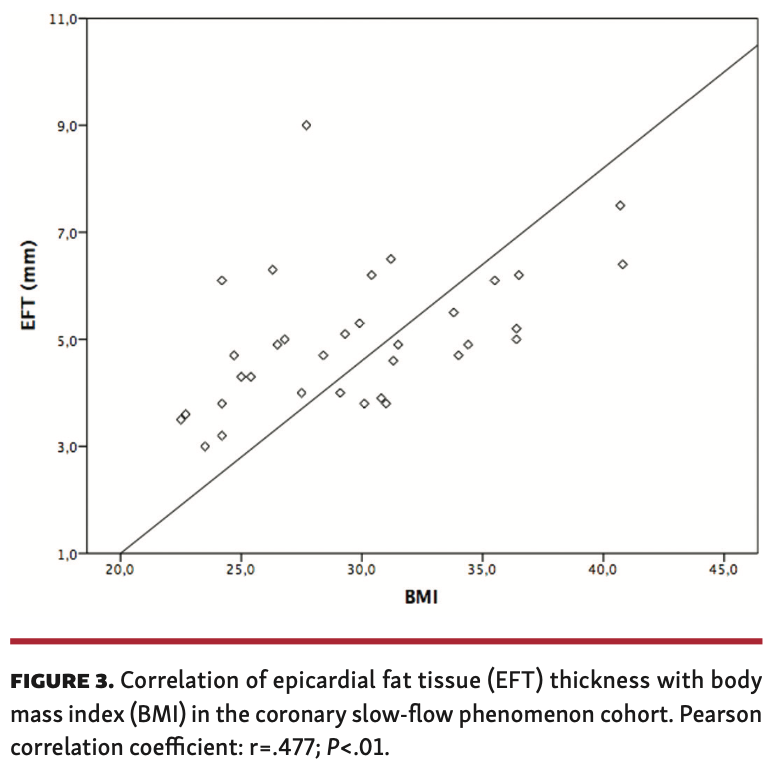
Association of CSFP and EFT. Echocardiographic measurements in at least 1 axis view were obtained for 34 patients. The median EFT thickness in the CSFP cohort was 4.9 mm (IQR, 4.0-6.1 mm) vs 3.9 mm (IQR, 3.1-4.9 mm) in the control group (P<.001) (Figure 2). When PLAX and PSAX views were considered separately, a difference in EFT between the groups was most evident in the PLAX view (4.8 mm [IQR, 4.0-5.6 mm] vs 3.7 mm [IQR, 3.0-4.8 mm]; P<.001) (Table 2). EFT thickness correlated significantly with BMI (r=.477; P<.01) in the CSFP group whereas EFT and BMI did not correlate at all in the control cohort (r=.094; P=.60) (Figures 3 and 4). Age had no impact on EFT thickness in the CSFP group (r=.117; P=.51); however, in the control cohort, age correlated significantly with EFT thickness (r=.468; P<.01). There was no relevant association between EFT thickness and cardiovascular risk factors (Table 3).
Follow-up. Median follow-up time was 23 months (IQR, 12-48 months) for the CSFP cohort and 35 months (IQR, 25-42 months) for the control cohort. Follow-up data were available for 43 patients in the CSFP cohort (90%) and for all patients in the control cohort. No patient died in the CSFP cohort, whereas 1 patient died in the control cohort. There was no occurrence of acute myocardial infarction during follow-up in either cohort.
Discussion
The major finding of the present study is the association of EFT with CSFP. Patients with CSFP had thicker EFT than patients with normal coronary artery flow. Furthermore, we identified male predominance, history of smoking, and elevated BMI as contributing clinical factors of CSFP. The findings of the case-control studies by Beltrame et al and Hawkins et al are consistent with our findings.1,2 In Beltrame’s analysis, male sex and nicotine use were found more frequently in CSFP patients than in the control group. Hawkins et al identified male sex and higher BMI as predictors of CSFP, although smoking habits did not correlate. They demonstrated a dependent vessel involvement of CSFP with increase in BMI. This positive correlation of BMI and CSFP is supported by findings from Yilmaz et al.13 We can confirm this finding for those patients with 3-vessel involvement compared with 1- and 2-vessel involvement, although this was not statistically significant. However, a direct comparison of BMI with the control cohort in our study was impossible since BMI was one of the matching factors between the groups.
The distribution pattern of CSFP observed in the LAD, circumflex, and RCA is comparable with previously published studies,1 with the LAD the most affected vessel and the RCA the second-most affected vessel. However, Hawkins et al found an equal distribution of vessel involvement for CSFP patients.2 The authors explain this finding with the higher burden of cardiovascular risk factors in their cohort, surmising that it leads to a more “diffuse” involvement of coronary arteries in slow flow.
During the past few years, several studies reported a positive relationship between the extent of EFT and the prevalence of CAD.14,15 In a meta-analysis by Xu et al, EFT thickness and volume were found to be significantly higher in patients with CAD than in subjects without CAD.16 Further data exist showing a strong association between CAD severity and EFT thickness14,17 and a positive correlation between EFT thickness and adverse cardiovascular outcomes in CAD patients.18 The exact causal pathophysiology is still not fully understood, but as a metabolic source epicardial fat produces proatherogenic and proinflammatory cytokines that can potentially interact with the myocardium and coronary arteries via paracrine and vasocrine pathways, possibly promoting coronary atherosclerosis.19,20 The fact that EFT and the myocardium share the same microcirculation due to the lack of separating fascias supports this theory.9,10 Nevertheless, these findings cannot simply be transferred to CSFP patients. Since no patient suffered a myocardial infarction during follow-up, it remains debatable whether CSFP can be considered a preliminary stage of CAD. The finding that EFT is thicker in CSFP patients raises the question of whether proinflammatory factors released by EFT potentially induce a different pathomechanism leading to endothelial dysfunction, which results in slow flow but not in atherosclerosis. The finding that BMI and EFT thickness correlate in our CSFP cohort but not in the control cohort leads to the assumption that weight loss in those patients could be a therapeutic target. In this context, data exist showing EFT reduction after weight loss.21-23 However, whether a decrease in EFT thickness improves coronary flow has not yet been investigated. Furthermore, statins were found to have a diminishing effect on EFT, and myocardial perfusion improved after a short- and mid-term statin course in CSFP patients.24-26 Whether this effect is due to a decrease in EFT or possibly based on the anti-inflammatory ability of statins (or even both) is not clear.
To our knowledge, this is the first study to investigate a relation between EFT and CSFP. Further studies are warranted to demonstrate the paracrine effects of EFT on coronary arteries, which would strengthen our theory.
Study limitations. Our study has several limitations. First, there is no consensus on definite diagnostic criteria for CSFP, leading to the extensive variety of prevalence in the literature. We followed the criteria established by Beltrame et al, as to our knowledge, they were the first and only authors who have proposed diagnostic criteria in this field.11 Given the lack of standardized and uniform criteria for CSFP, comparisons between the different studies are challenging. The prevalence of 0.13% in the present study is one of the lowest in the literature. As this is a retrospective analysis based on an electronic search of medical records for distinct terms describing CSFP, it is possible that the prevalence of CSFP might have been underestimated, as it might not always have been thoroughly described in the corresponding angiographic record. Finally, this a single-center study with a small sample size, making transferability difficult.
Conclusion
EFT is thicker in patients with CSFP than in a matched control group. Thus, EFT might serve as a therapeutic target, but more studies with larger sample sizes are needed to prove a causal relationship and to further elucidate the pathophysiology of this rare disease.
From the 1Department of Cardiology, Kerckhoff Heart and Thorax Center, Bad Nauheim, Germany; 2German Centre for Cardiovascular Research (DZHK), partner site RheinMain, Germany; and 3University of Giessen, Medical Department I, Cardiology, Giessen, Germany.
Funding: The control group of this research project is based on a cohort that is part of the Kerckhoff Biomarker Registry (BioReg) that is financially supported by the Kerckhoff Heart Research Institute (KHFI) and the German Center for Cardiovascular Research e.V. (DZHK). The sponsors had no influence on the study design, statistical analyses or draft of the paper.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Final version accepted June 20, 2020.
Address for correspondence: Maren Weferling, MD, Department of Cardiology, Kerckhoff Heart and Thorax Center, Bad Nauheim, Germany. Email: m.weferling@kerckhoff-klinik.de
- Beltrame JF, Limaye SD, Horowitz JD. The coronary slow flow phenomenon — a new coronary microvascular disorder. Cardiology. 2002;97:197-202.
- Hawkins BM, Stavrakis S, Rousan TA, et al. Coronary slow flow — prevalence and clinical correlations. Circ J. 2012;76:936-942.
- Mangieri E, Macchiarelli G, Ciavolella et al. Slow coronary flow: clinical and histopathological features in patients with otherwise normal epicardial coronary arteries. Cathet Cardiovasc Diagn. 1996;37:375-381.
- Tambe AA, Demany MA, Zimmerman HA, et al. Angina pectoris and slow flow velocity of dye in coronary arteries: a new angiographic finding. Am Heart J. 1972;84:66-71.
- Iacobellis, G, Conradi D, Scharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationsships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536-543.
- Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253-261.
- Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol. 2013;101:e18-e28.
- Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611-3615.
- Iacobellis G, Binco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450-457.
- Fricke AC, Iacobellis G. Epicardial adipose: clinical biomarker of cardiometabolic risk. Int J Mol Sci. 2019;20:5989.
- Beltrame JF. Defining the slow flow phenomenon. Circ J. 2012;76:818-820.
- Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311-1319.
- Yilmaz H, Demir I, Unar Z. Clinical and coronary angiographic characteristics of patients with coronary slow flow. Acta Cardiol. 2008;63:579-584.
- Verma B, Katyal D, Patel A. et al. Relation of systolic and diastolic epicardial adipose tissue thickness with presence and severity of coronary artery disease (the EAT CAD study). J Family Med Prim Care. 2019;8:1470-1475.
- Shemirani H, Khoshavi M. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease — an observational study. Anadolu Kardiyol Derg. 2012;12:200-205.
- Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis. 2012;23:227-233.
- Erkan AF, Tanindi A, Kocaman SA, Ugurlu M, Tore HF. Epicardial adipose tissue thickness is an independent predictor of critical and complex coronary artery disease by Gensini and Syntax scores. Tex Heart Inst J. 2016;43:29-37.
- Morales-Portano JD, Peraza-Zaldivar JA, Suarez-Cuenca JA, et al. Echocardiographic measurements of epicardial adipose tissue and comparative ability to predict adverse cardiovascular outcomes in patients with coronary artery disease. Int J Cardiovasc Imag. 2018;34:1429-1437.
- Singh N, Singh H, Khanijoun HK, Iacobellis G. Echocardiographic assessment of epicardial adipose tissue — a marker of visceral adiposity. McGill J Med. 2007;10:26-30.
- Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43:1651-1654.
- Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106:5-11.
- Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev. 2015;16:406-415.
- Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008;16:1693-1697. Epub 2008 May 1.
- Park JH, Park YS, Kim YJ, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimib. J Cardiovasc Ultrasound. 2010;18:121-126.
- Cakmak M, Tanriverdi H, Cakmak N, et al. Simvastatin may improve myocardial perfusion abnormality in slow coronary flow. Cardiology. 2008;110:39-44.
- Caliskan M, Erdogan D, Gullun H, et al. Effects of atorvastatin on coronary flow reserve in patients with coronary slow flow. Clin Cardiol. 2007;30:475-479.