Adverse Events After Left Atrial Appendage Closure: Lessons Learned From the Manufacturer and User Facility Device Experience (MAUDE) Database
Abstract: Left atrial appendage (LAA) closure devices are alternative treatments recently approved for patients with atrial fibrillation. Due to the novelty of these devices, limited postapproval surveillance data on LAA closure devices have been published. Thus, we analyzed the United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database to report these findings. The primary endpoint was final event outcome, and secondary endpoints included management strategies of reported events.
Key words: atrial fibrillation, left atrial appendage closure
Atrial fibrillation affects between 2.7 million and 6.1 million people in the United States,1,2 with an associated risk of stroke in 5% of non-anticoagulated patients every year. For decades, oral anticoagulation with vitamin K antagonists have been useful in the prevention of stroke risk from atrial fibrillation;3-5 however, monitoring and adjustment of coagulation levels are tedious and not ideal for outpatient clinical practice.6,7 Novel oral anticoagulants are also effective in stroke prevention yet suffer from the high cost and scarcity of antidotes.8 The left atrial appendage (LAA), in particular, has been implicated as a high-risk area of clot formation in this setting. LAA closure devices are alternative treatments recently approved for patients with atrial fibrillation. In early randomized trials, LAA closure devices were found comparable to warfarin in the prevention of strokes for affected individuals.9 Furthermore, in patients with increased bleeding or stroke risk, LAA closures provided better outcomes for hemorrhagic stroke, cardiovascular-related death, and non-procedural bleeding than typical vitamin K antagonists.10 Nevertheless, due to the novelty of these devices, limited postapproval surveillance data on LAA closure devices have been published. Thus, we analyzed the United States Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database to report these findings.
Methods
The MAUDE database was established by the FDA to record adverse events associated with the use of medical devices.12 Requirements for reporting adverse events are dependent on the party involved; device manufacturers and user facilities are obligated to report events, while clinicians, patients, and consumers may voluntarily report. Medical device reports (MDRs) are uploaded monthly and include a description of the occurred event. Through these descriptions, events are classified as malfunction, injury, death, or other.
The MAUDE database was queried by four independent reviewers from April 2015 to December 2018 for device reports of only FDA-approved LAA closure devices. The primary endpoint was final event outcomes. Secondary endpoints included management strategies of reported events.
Results
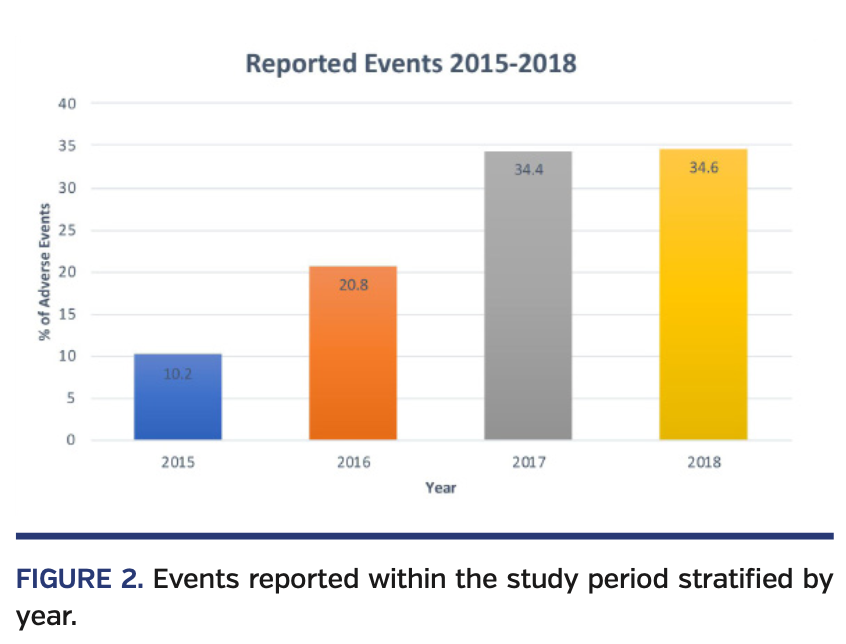
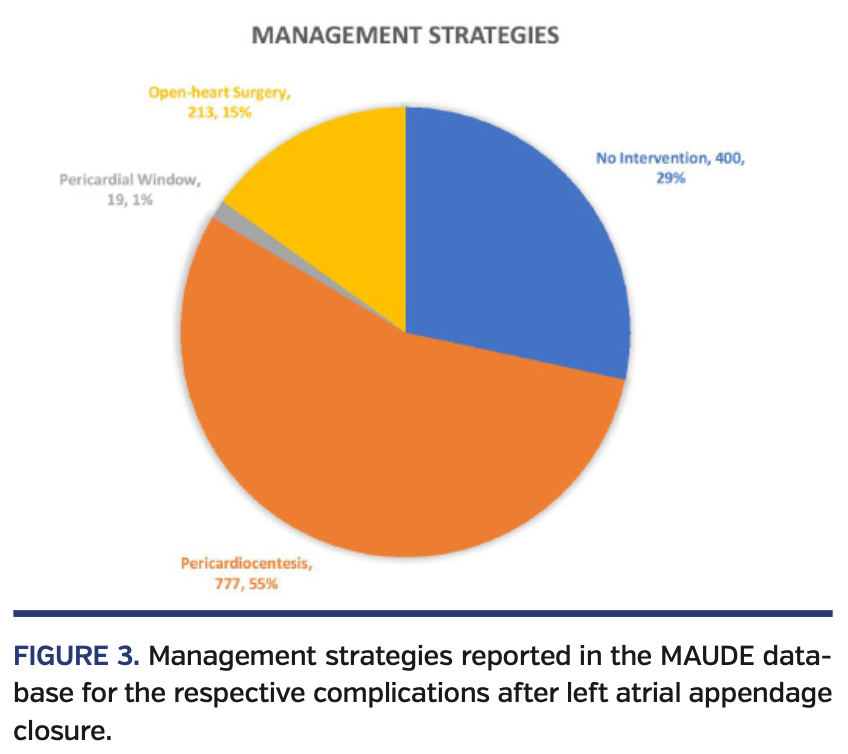
Between April 2015 and December 2018, a total of 2974 medical device reports from an FDA-approved LAA closure device were recorded. From 2015 to 2018, reported events rose as follows: 2015, 10.2%; 2016, 20.8%; 2017, 34.4%; and 2018, 34.6% (Figure 1). Most commonly reported morbid events were pericardial effusion (n = 1257; 42%), thrombus formation (n = 323; 11%), cerebrovascular accidents (n = 170; 5.7%), device embolization (n = 149; 5%), and significant device leaks requiring hospitalization (n = 75; 2.5%). Adverse morbid events resulted in mortality for 211 patients (7%) (Figure 2). Management strategies in patients with pericardial effusion included no intervention (n = 400; 32%), pericardiocentesis (n = 777; 62%), pericardial window (n = 19; 1.5%), and open-heart surgery (n = 213; 16.9%); single-event concomitant interventions were recorded as unique interventions in the abovementioned management strategies (n = 152). Sixty-four of the managed patients required intensive unit level of care. Other events reported (n = 789) were device-system related or non-morbid complications (Figure 3).
Discussion
In this study of a nationwide registry of reported events after LAA closure, a commonly reported event, pericardial effusion, was almost four times as prevalent as the next leading complication. A curious observation was made in the 11% of thrombus formations; this unintended outcome is in direct contradiction to the purpose of the Watchman device (Boston Scientific), which may have contributed to the 5.7% of cerebral vascular accidents.
When analyzing the data by year, later years had significantly higher rates of reported events. It is difficult to put this number into context, as many simple factors affect this number; it is expected that as implantation becomes prevalent, more LAA closures will be performed by newly trained clinicians. Conversely, as clinicians gain more experience and techniques are optimized, the complication rate would be expected to drop. Consequently, data will need to be analyzed from all implantations, as training becomes more widespread, to delineate current standings.
Death occurred in 7% of adverse events reported. This seemingly high number can be troubling to some operators, but must be viewed in the context of the overall number of implantations for the assessment of risk and benefits. Management strategies are somewhat optimistic for non-fatal adverse events noted. While pericardial effusions were undoubtedly common, 32% of events were controlled with observation and medical management. Furthermore, 62% were managed with pericardiocentesis, and less than 19% of adverse events required open surgical intervention, which negates the benefit of the initial minimally invasive procedure.
Overall, more data are needed to understand the risks associated with the use of LAA closure devices. As these procedures become more refined and clinicians more experienced, complication rates are expected to drop. Thus, it is incumbent upon the manufacturers to continually analyze, optimize, and simplify their procedures for better outcomes. Furthermore, significant responsibility falls to the clinician in reporting adverse events; both acute and long-term complications will be better depicted with comprehensive reporting.
Study limitations. LAA closure devices are still relatively new in the United States, which limits this investigation to short-term findings. Other limitations of this study stem from the use of the MAUDE database. As a partially voluntary reporting system, duplicate MDRs may be seen when the manufacturer, the clinician, and the patient all report the same event. Additionally, delayed complications may be omitted as these events would require the patient or clinician to report to the FDA. Furthermore, device failures or complications may be improperly attributed to the manufacturer when reported voluntarily. Moreover, the presented data should be interpreted with caution, as they represent the number of events encountered only and not rates or incidence. Since the MAUDE database does not provide the total number of LAA closure procedures performed, the incidence or prevalence of these complications cannot be inferred from the reported data. Nevertheless, review of these data provides invaluable insight into the most commonly encountered complications during LAA closure procedures. Finally, the FDA collects data to be used in the MAUDE database, but does not verify its integrity.
Conclusion
Analysis of the MAUDE database establishes a typical list of complications associated with the use of an LAA closure device. Through the continued training, use, and refinement of LAA closure procedures, complication rates can be monitored and used for the improvement of device performance.
Impact on daily practice. LAA closure devices present a novel approach to reduce the risk of stroke in the setting of atrial fibrillation. While LAA closure devices possess beneficial characteristics, they are not without concomitant drawbacks. Thus, the use of LAA closure devices should be closely monitored to assess their efficacy in clinical use.
From the 1Cardiovascular Research Unit, RWJ Barnabas Health. NBIMC, Newark, New Jersey; and 2Department of Medicine, The Brooklyn Hospital Center, Brooklyn, New York.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript accepted February 5, 2020.
Address for correspondence: Alexis Kofi Okoh, MD, Cardiovascular Research Unit, RWJ Barnabas Health Heart Centers, Newark Beth Israel Medical Center, 201 Lyons Avenue, Suite G5, Newark, NJ 07112. Email: alexis.okoh@rwjbh.org
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370-2375.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
- Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL, Manning WJ. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:429S-456S.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
- Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927-934.
- Piccini JP, Hernandez AF, Zhao X, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54:1280-1289.
- Rogers K, Shelton M, Finks S. Reversal agents for direct oral anticoagulants: understanding new and upcoming options. Cardiol Rev. 2016;24:310-315.
- Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Am Heart J. 2006;151:956-961.
- Holmes DR, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614-2623.
- Waks JW, Manning WJ. Left atrial appendage closure to reduce the risk of thromboembolic complications in atrial fibrillation: pay now and possibly pay later? J Am Coll Cardiol. 2015;65:2624-2627.
- MAUDE - Manufacturer and User Facility Device Experience. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed July 15, 2020.