Ad-hoc Percutaneous Coronary Intervention and Transcatheter Aortic Valve Implantation as a Combined Transfemoral Procedure
ABSTRACT: Coronary artery disease has been reported in more than 50% of patients with severe aortic stenosis above the age of 70 years. Combined surgical aortic valve replacement (SAVR) and coronary artery bypass grafting (CABG) is associated with a higher operative risk. Concomitant coronary artery disease also increases the procedural risk of transcatheter aortic valve implantation (TAVI), and hence, a combined strategy for treating both entities needs to be carefully considered. Data regarding TAVI and percutaneous coronary intervention (PCI) as a combined percutaneous procedure are scarce. We report the case of an 84-year-old woman who presented with non-ST segment elevation myocardial infarction and impending pulmonary edema who was diagnosed with severe aortic stenosis and two-vessel coronary artery disease. Because of an elevated logistic Euroscore of 25% and her unstable presentation, percutaneous coronary revascularization and TAVI were successfully performed in a combined percutaneous transfemoral procedure. She had a smooth recovery and rehabilitation period with significant improvement in her symptoms and functional capacity. Thirty-day follow-up, including transthoracic echocardiography and cardiac magnetic resonance imaging, showed a well-functioning prosthetic valve and no signs of residual myocardial ischemia. We therefore conclude that combined PCI and TAVI is feasible and can be associated with good clinical outcomes in selected cases. Further data and experience are needed to evaluate this strategy.
J INVASIVE CARDIOL 2011;23:E102–E105
_____________________________________
Patients with symptomatic severe aortic stenosis (AS) and associated coronary artery disease (CAD) usually undergo combined surgical aortic valve replacement (SAVR) and coronary artery bypass grafting (CABG), though this is associated with a higher operative risk compared to isolated SAVR. Trans-catheter aortic valve implantation (TAVI) has recently emerged as a therapeutic option for patients with severe calcified AS for whom surgical valve replacement is not suitable, especially those considered to be at high surgical risk. Concomitant CAD is present in at least 50% of TAVI patients and has been associated with worse in-hospital and intermediate-term outcomes,1,2 but so far, there are no data regarding the feasibility and outcome of performing TAVI and percutaneous coronary intervention (PCI) in a combined percutaneous procedure, and a therapeutic strategy for the percutaneous handling of both entities has not been established. We report a case where both TAVI and PCI were simultaneously performed in a patient presenting with an acute coronary syndrome.
 Case Report. An 84-year-old, diabetic, hypertensive and obese woman presented with a non-ST segment elevation myocardial infarction and impending pulmonary edema. She gave history of recently worsening dyspnea on minimal exertion (New York Heart Association class III) together with effort angina on mild exertion with marked limitation of her ordinary activity (Canadian Cardiovascular Society class III) over the previous 6 months. Cardiac examination revealed the presence of a harsh ejection systolic murmur over the cardiac base radiating to the neck (grade IV), and bilateral fine rales were audible over the basal and mid fields of her lungs, denoting pulmonary congestion. Her electrocardiogram showed ST-segment depression and T-wave inversion in the lateral chest leads (V3–V6) together with leads I, aVL and lead II (Figure 1), and her chest x-ray confirmed the presence of severe pulmonary congestion (Figure 2A). Troponin T, total creatine kinase (CK) and CK-MB were elevated. An echocardiography done a few months before admission showed evidence of severe aortic stenosis, yet the patient
Case Report. An 84-year-old, diabetic, hypertensive and obese woman presented with a non-ST segment elevation myocardial infarction and impending pulmonary edema. She gave history of recently worsening dyspnea on minimal exertion (New York Heart Association class III) together with effort angina on mild exertion with marked limitation of her ordinary activity (Canadian Cardiovascular Society class III) over the previous 6 months. Cardiac examination revealed the presence of a harsh ejection systolic murmur over the cardiac base radiating to the neck (grade IV), and bilateral fine rales were audible over the basal and mid fields of her lungs, denoting pulmonary congestion. Her electrocardiogram showed ST-segment depression and T-wave inversion in the lateral chest leads (V3–V6) together with leads I, aVL and lead II (Figure 1), and her chest x-ray confirmed the presence of severe pulmonary congestion (Figure 2A). Troponin T, total creatine kinase (CK) and CK-MB were elevated. An echocardiography done a few months before admission showed evidence of severe aortic stenosis, yet the patient 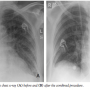 refused further invasive therapy at that time. A new echocardiography revealed good systolic function with an estimated ejection fraction of 60%, concentric left ventricular hypertrophy with no evidence of segmental wall motion abnormality, and a heavily calcified aortic valve with a mean and peak pressure gradient of 65 mmHg and 110 mmHg, respectively, and an estimated aortic valve area of 0.6 cm2.
refused further invasive therapy at that time. A new echocardiography revealed good systolic function with an estimated ejection fraction of 60%, concentric left ventricular hypertrophy with no evidence of segmental wall motion abnormality, and a heavily calcified aortic valve with a mean and peak pressure gradient of 65 mmHg and 110 mmHg, respectively, and an estimated aortic valve area of 0.6 cm2.
With a calculated logistic Euroscore of 25% (based on the patient’s age and sex, her presentation with an acute myocardial infarction and her critical pre-operative state), the decision was made for an invasive assessment of the coronary status with the possibility of performing PCI and TAVI in a combined percutaneous approach. The procedure was performed under conscious sedation and angiographic guidance. Coronary angiography revealed two-vessel disease, with  an 80% left circumflex artery (LCX) stenosis and an ostial 80% right coronary artery (RCA) stenosis (Figures 3A and 4A). Aortic root angiography revealed trivial aortic regurgitation and an aortic annular diameter of 24 mm, and peripheral angiography showed adequately-sized common femoral and iliac arteries with no significant stenosis or kinking. Successful percutaneous coronary intervention with implantation of two zotarolimus-eluting stents was achieved in
an 80% left circumflex artery (LCX) stenosis and an ostial 80% right coronary artery (RCA) stenosis (Figures 3A and 4A). Aortic root angiography revealed trivial aortic regurgitation and an aortic annular diameter of 24 mm, and peripheral angiography showed adequately-sized common femoral and iliac arteries with no significant stenosis or kinking. Successful percutaneous coronary intervention with implantation of two zotarolimus-eluting stents was achieved in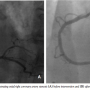 both the LCX and RCA (Figures 3B and 4B). Thereafter, an 18 Fr sheath was advanced into the right femoral artery under fluoroscopic guidance. The calcified stenotic aortic valve was crossed by a straight Terumo wire (Terumo, Tokyo, Japan) with the help of an Amplatz left catheter. A pigtail catheter was then advanced into the left ventricle and pressure tracings for left ventricle and aorta were simultaneously measured (Figure 5A). Then, a stiff Meier back-up wire (Boston Scientific, Natick, Massachusetts) was placed into the left ventricle and balloon aortic valvuloplasty was performed with a 22 x 60 mm Tyshak II balloon
both the LCX and RCA (Figures 3B and 4B). Thereafter, an 18 Fr sheath was advanced into the right femoral artery under fluoroscopic guidance. The calcified stenotic aortic valve was crossed by a straight Terumo wire (Terumo, Tokyo, Japan) with the help of an Amplatz left catheter. A pigtail catheter was then advanced into the left ventricle and pressure tracings for left ventricle and aorta were simultaneously measured (Figure 5A). Then, a stiff Meier back-up wire (Boston Scientific, Natick, Massachusetts) was placed into the left ventricle and balloon aortic valvuloplasty was performed with a 22 x 60 mm Tyshak II balloon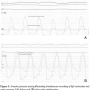 (NuMED, Hopkinton, New York) under rapid pacing, followed by implantation of a 29 mm Medtronic CoreValve prosthesis (Medtronic Parkway, Minneapolis, Minnesota). Final rest gradient across the aortic valve was 5 mmHg with only mild aortic regurgitation (Figure 5B). Vascular closure was achieved percutaneously with the Perclose vascular closure device (Abbott Vascular). The whole procedure duration was 48 minutes with a total of 250 ml of contrast material used.
(NuMED, Hopkinton, New York) under rapid pacing, followed by implantation of a 29 mm Medtronic CoreValve prosthesis (Medtronic Parkway, Minneapolis, Minnesota). Final rest gradient across the aortic valve was 5 mmHg with only mild aortic regurgitation (Figure 5B). Vascular closure was achieved percutaneously with the Perclose vascular closure device (Abbott Vascular). The whole procedure duration was 48 minutes with a total of 250 ml of contrast material used.
The patient was kept in the intensive care unit for 3 more days and then transferred to the department for further management and rehabilitation. She reported rapid relief of her symptoms with marked improvement in her functional capacity (Figure 2B). Dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) was advised for 6 months, and aspirin was prescribed lifelong. Thirty-day follow-up echocardiography revealed a well functioning prosthetic aortic valve with an estimated valve area of 2.1 cm2 and a mean gradient of 12 mmHg. Cardiac magnetic resonance was performed for evaluating the CoreValve function and revealed mild aortic regurgitation with a retrograde volume of 5.96 ml (Figure 6). At 6 months, the patient continued to be symptom-free and the prosthetic aortic valve was functioning appropriately.
prosthetic aortic valve with an estimated valve area of 2.1 cm2 and a mean gradient of 12 mmHg. Cardiac magnetic resonance was performed for evaluating the CoreValve function and revealed mild aortic regurgitation with a retrograde volume of 5.96 ml (Figure 6). At 6 months, the patient continued to be symptom-free and the prosthetic aortic valve was functioning appropriately.
Discussion. The association of coronary artery and aortic valve disease is getting more common due to the evolution in the epidemiology of valvular diseases where degenerative lesions are now the most common cause of aortic valve disease in the Western world.3 There are also data suggesting that calcific AS and coronary atherosclerosis might share a similar pathophysiology.4 Significant CAD has been reported in more than 50% of patients with severe AS above 70 years of age, and an even higher incidence was reported in patient series belonging to older age groups.4 Hence, the combined treatment of both entities is a common problem in today’s practice.
Many series have reported immediate and late results of combined valvular and coronary surgery in patients with both aortic and coronary disease and compared these results with those obtained after isolated SAVR in patients with AS without coronary lesions. These comparative studies6–11 most often reported higher perioperative mortality rates after combined surgery than after SAVR alone.
TAVI has recently been introduced as a therapeutic option for patients with severe symptomatic AS and a high or unacceptable perioperative risk. Treating concomitant CAD prior to TAVI appears to be reasonable, as severe CAD might have a negative impact on the safety of the TAVI procedure, especially because of the need for rapid pacing and anesthesia. Also, concerns about access to the coronary arteries after TAVI argue for a PCI prior to TAVI,12 though some initial reports suggest the feasibility of PCI following valve implantation.13 However, data about a combined percutaneous approach such as the one presented are lacking. In contrast to surgery, patients with CAD who are being prepared for TAVI do not commonly get treated for both pathologies in the same setting. A thorough invasive assessment with or without PCI usually precedes valve implantation, which is a more demanding intervention and still requires at least a sedo-analgesia and large-bore catheters. In addition, the aortic annulus has to be sized pre-procedurally with transoesophageal echocardiography or computed tomography (CT), and the femoral and iliac arteries have to be evaluated for adequate size and morphology, usually with CT angiography. Both parameters were invasively assessed in our case because of the acute setting. In a case like the one we are presenting, where a high-risk patient acutely presents with symptoms and signs that could be attributed to both coronary and valvular pathologies, a combined ad-hoc percutaneous approach to both disease entities — if feasible — is undoubtedly an evolution in disease management. So far, such patients would have only two valid options: an emergency combined surgical approach with all related operative risks, or a combined PCI and balloon valvuloplasty, which would only offer a temporary solution to the problem.
Conclusion. Although data regarding the outcome of combined TAVI with PCI are still lacking, the case we present suggests that such a combined ad-hoc percutaneous procedure is feasible, and may provide a valid treatment modality for selected high-risk patients with both coronary artery and aortic valve disease.
References
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic bioprosthesis european outcome (SOURCE) registry. A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62–69.
- Dewey TM, Brown DL, Herbert MA, et al. Effect of concomitant coronary artery disease on procedural and late outcomes of transcatheter aortic valve implantation. Ann Thorac Surg 2010;89:758–767.
- Lung B. Interface between valve disease and ischemic heart disease. Heart 2000;84:347–352.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630–634.
- Akins CW, Daggett WM, Vlahakes GJ, et al. Cardiac operations in patients 80 years old and older. Ann Thorac Surg 1997;64:606–614.
- Mullany CJ, Elveback LR, Frye RL, et al. Coronary artery disease and its management: Influence on survival in patients undergoing aortic valve replacement. J Am Coll Cardiol 1987;10:66–72.
- Lytle BW, Cosgrove DM, Gill CC, et al. Aortic valve replacement combined with myocardial revascularization. J Thorac Cardiovasc Surg 1988;95:402–414.
- Czer LS, Gray RJ, Stewart ME, et al. Reduction in sudden late death by concomitant revascularization with aortic valve replacement. J Thorac Cardiovasc Surg 1988;95:390–401.
- Lund O, Nielsen TT, Pilegaard HK, et al. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 1990;100:327–337.
- Iung B, Drissi MF, Michel PL, et al. Prognosis of valve replacement for aortic stenosis with or without coexisting coronary heart disease: A comparative study. J Heart Valve Dis 1993;2:430–439.
- Flameng WJ, Herijgers P, Szécsi J, et al. Determinants of early and late results of combined valve operations and coronary artery bypass grafting. Ann Thorac Surg 1996;61:621–628.
- Figulla HR, Cremer J, Walther T, et al. Positionspapier zur Kathetergefuehrten Aortenklappenintervention. Kardiologe 2009;3:199–206.
- Geist V, Sherif MA, Khattab AA. Successful percutaneous coronary intervention after implantation of a CoreValve percutaneous aortic valve. Catheter Cardiovasc Interv 2009;73:61–67.
______________________________
From the Heart Center, Segeberger Kliniken GmbH (Academic Teaching Hospital of the University of Kiel), Bad Segeberg, Germany.
The authors report no conflicts of interest regarding the content herein.
Manuscript submitted August 9, 2010, provisional acceptance given August 25, 2010, final version accepted September 21, 2010.
Address for correspondence: Mohamed Abdel-Wahab, MD, Herzzentrum, Segeberger Kliniken GmbH, Am Kurpark 1, 23795 Bad Segeberg — Germany. Email: mohamed.abdel-wahab@segebergerkliniken.de












