Acquired Left Ventricular to Left Atrial Fistula – Not Mitral Regurgitation! Transcutaneous Closure with Amplatzer Device
ABSTRACT: Acquired left ventricle (LV) to left atrial (LA) fistula is a very rare complication following aortic valve replacement (AVR). This can usually be surgically repaired but the risk of re-operation is high due to repeat sternotomy and also due to other comorbidities usually seen in this population. We report a case presenting with recurrent episodes of left ventricular failure 10 years following bioprosthetic aortic valve replacement and who was diagnosed to have a communication between the LV and the LA on transesophageal echocardiography (TEE). This was treated percutaneously with an Amplatzer duct occluder (ADO) device (AGA Medical Corp.) as she was considered to be a high surgical risk.
J INVASIVE CARDIOL 2012;24(1):E16-E18
Key words: left ventricular fistula, Amplatzer device, aortic valve replacement, mitral regurgitation
__________________________________________
 Acquired left ventricle (LV) to left atrial (LA) fistula is a very rare complication following aortic valve replacement (AVR). This can usually be surgically repaired but the risk of re-operation is high due to repeat sternotomy and also due to other comorbidities usually seen in this population. We report a case presenting with recurrent episodes of left ventricular failure 10 years following bioprosthetic aortic valve replacement and who was diagnosed to have a communication between the LV and the LA on transesophageal echocardiography (TEE). This was treated percutaneously with an Amplatzer duct occluder (ADO) device (AGA Medical Corp.) as she was considered to be a high surgical risk.
Acquired left ventricle (LV) to left atrial (LA) fistula is a very rare complication following aortic valve replacement (AVR). This can usually be surgically repaired but the risk of re-operation is high due to repeat sternotomy and also due to other comorbidities usually seen in this population. We report a case presenting with recurrent episodes of left ventricular failure 10 years following bioprosthetic aortic valve replacement and who was diagnosed to have a communication between the LV and the LA on transesophageal echocardiography (TEE). This was treated percutaneously with an Amplatzer duct occluder (ADO) device (AGA Medical Corp.) as she was considered to be a high surgical risk.
Case Report. An 86-year-old female who had a 20 mm Tissuemed Porcine AVR (Aspire) in 2000 presented with increasing shortness of breath 4 years following her surgery. A transthoracic echocardiogram (TTE) reported severe eccentric mitral regurgitation and it was suspected there could be a perforation in the anterior mitral valve leaflet. She did not want to undergo repeat surgery and, in view of her comorbidities, was managed with anti-failure medication.
 In 2010 she had recurrent admissions with pulmonary edema. TEE was performed and revealed a shunt between the LV and the left atrium at the aortomitral junction, which on TTE falsely appeared to be an eccentric jet of mitral regurgitation. There was also mild stenosis and moderate aortic regurgitation noted. In view of her high risk for surgery, she was referred for percutaneous device closure. There were no vegetations and no evidence of infection. She was on full anti-failure medication and on warfarin because of atrial fibrillation.
In 2010 she had recurrent admissions with pulmonary edema. TEE was performed and revealed a shunt between the LV and the left atrium at the aortomitral junction, which on TTE falsely appeared to be an eccentric jet of mitral regurgitation. There was also mild stenosis and moderate aortic regurgitation noted. In view of her high risk for surgery, she was referred for percutaneous device closure. There were no vegetations and no evidence of infection. She was on full anti-failure medication and on warfarin because of atrial fibrillation.
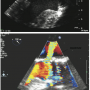
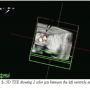
Periprocedural TEE showed the defect between the LV and the LA measured 7 mm (Figures 1A and B) and 2 color flow jets were visualized suggesting 2 leaks adjacent to each other. The defect was also clearly visualized on 3D TEE (Figure 2). TEE guidance is very useful in this situation to assess the anatomy clearly and guide device closure percutaneously.
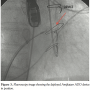 The procedure was carried out under general anesthesia via the right femoral vein and the left femoral artery. Due to anticoagulation, an arterio-arterial circuit was initially created by crossing the fistula from the LV into the LA using a Terumo wire. The wire crossed the mitral valve and reached the ascending aorta, thus forming a circuit. This approach failed, however, due to the tight course making it unsuitable for a delivery sheath. A veno-arterial circuit was then created, crossing the fistula from the LV and snaring the Terumo wire in the LA, having performed a transseptal puncture. This circuit allowed balloon sizing of the defect by introducing a 7 mm balloon through the femoral vein and also allowed the delivery sheath through the same access. A 10 x 8 mm ADO (Amplatzer) was delivered and deployed across the fistula using fluoroscopy, but mainly TEE, for accurate placement as shown in Figure 3. Once the device was confirmed in an optimal position and secure without causing LV outflow obstruction or mitral regurgitation on cross-sectional and 3D TEE, it was released. There were no procedural complications. Postoperatively, she had transient renal dysfunction from which she recovered. She was placed on aspirin.
The procedure was carried out under general anesthesia via the right femoral vein and the left femoral artery. Due to anticoagulation, an arterio-arterial circuit was initially created by crossing the fistula from the LV into the LA using a Terumo wire. The wire crossed the mitral valve and reached the ascending aorta, thus forming a circuit. This approach failed, however, due to the tight course making it unsuitable for a delivery sheath. A veno-arterial circuit was then created, crossing the fistula from the LV and snaring the Terumo wire in the LA, having performed a transseptal puncture. This circuit allowed balloon sizing of the defect by introducing a 7 mm balloon through the femoral vein and also allowed the delivery sheath through the same access. A 10 x 8 mm ADO (Amplatzer) was delivered and deployed across the fistula using fluoroscopy, but mainly TEE, for accurate placement as shown in Figure 3. Once the device was confirmed in an optimal position and secure without causing LV outflow obstruction or mitral regurgitation on cross-sectional and 3D TEE, it was released. There were no procedural complications. Postoperatively, she had transient renal dysfunction from which she recovered. She was placed on aspirin.
Discussion. Mitral-aortic intervalvular fibrosa is the junction between the left half of the noncoronary cusp and the adjacent third of the left coronary cusp of the aortic valve and the anterior mitral leaflet. This junctional zone between the mitral and aortic valves is formed by fibrous annular tissue and therefore is referred to as the mitral-aortic intervalvular fibrosa. This area may be involved in infections of either of these valves. It is a relatively avascular zone and is therefore more prone to become colonized with organisms.1 Pseudoaneurysm arising from the subaortic annular fibrous tissue is a rare but life threatening complication of aortic valve endocarditis.2
Chesler et al first described aneurysm of the mitral aortic intervalvular fibrosa in 1968 in a patient with aortic valve endocarditis.3 Other than an infective etiology, blunt trauma and weakness of the subvalvular annular structures, either congenital or secondary to aortic valve replacement, also have been reported as causes of aneurysm.4,5 If the aneurysm ruptures, a fistula is formed between the LV and LA. This can be difficult to detect on TTE and can be mistaken for an eccentric mitral regurgitation jet as reported in a case series.6 A high index of suspicion should prompt an early TEE, which is usually diagnostic.
There are reports of device closure of paravalvular leaks involving prosthetic mitral or aortic valves or ruptured sinus of Valsalva aneurysms using Amplatzer devices (AGA Medical).7,8 There is one case report of an aorta to left atrial fistula that was percutaneously closed with an Amplatzer occluder device (AGA).9 To our knowledge there have been no reported cases of a LV to LA fistula that have been closed percutaneously with this technique and this case shows that it is feasible. We elected to use an Amplatzer ADO device (AGA) as this has a 2 mm rim, which was placed on the LV side and was unlikely to interfere with the aortic or mitral valves. Moreover, a proximal disc was not necessary due to the marked pressure differential between the LV and the LA. Amplatzer devices are made from nitinol wires, which form a mesh with a predetermined shape and size. Some, including the ADO, have Dacron patches incorporated. These devices are very versatile and can be delivered through relatively small French sheaths due to the memory characteristics of the nitinol alloy.
The choice of the ADO has to do with the small rim. Because the LV pressure is much higher than the LA, we did not need a rim on the LA side as this could have interfered with the MV leaflets and was not necessary. The defect appeared round on 3D TEE and so the AVPIII, which are oblong, would not have been suitable. For the same reasons, in this particular location, using an ASD, VSD, or ADOII device would not have been ideal. It is also important to distinguish this LV-LA fistula from a paravalvular leak as in the latter there is a valve rim but in our case we had no rim around the mitral valve. A small rim would have also helped protect the aortic valve.
Transesophageal echocardiography with 3D (iE33, Phillips) is very useful in this situation. It provided us with the size, shape, and position of the defect as well as essential detail of the device required to close the defect and its relationship to the neighboring mitral and aortic valves. It was also useful in detecting residual leaks after device deployment.
Conclusion. In patients with AVR who develop a jet in the LA, one should consider the possibility of an LV to LA fistula as an alternative to mitral regurgitation, and a TEE is recommended. Percutaneous closure is feasible and we believe this is the first such case in the literature. It could be considered as the preferred approach even in less risky circumstances.
References
- Karalis DG, Bansal RC, Hauck AJ, et al. Transesophageal echocardiographic recognition of subaortic complications in aortic valve endocarditis. Clinical and surgical implications. Circulation. 1992 Aug;86(2):353-362.
- Tak T. Pseudoaneurysm of mitral-aortic intervalvular fibrosa. Clin Med Res. 2003 Jan;1(1):149-152.
- Chesler E, Korns ME, Porter GE, Reyes CN, Edwards JE. False aneurysm of the left ventricle secondary to bacterial endocarditis with perforation of the mitral-aortic intervalvular fibrosa. Circulation. 1968 Apr; 37(4):518-523.
- Matthews RV, French WJ, Criley JM. Chest trauma and subvalvular left ventricular aneurysms. Chest. 1989 Feb;95(2):474-476.
- Bansal RC, Graham BM, Jutzy KR, Shakudo M, Shah PM. Left ventricular outflow tract to left atrial communication secondary to rupture of mitral-aortic intervalvular fibrosa in infective endocarditis: diagnosis by transesophageal echocardiography and color flow imaging. J Am Coll Cardiol. 1990 Feb;15(2): 499-504.
- Ananthasubramaniam K. Clinical and echocardiographic features of aorto-atrial fistulas. Cardiovasc Ultrasound. 2005 Jan;3:1.
- Korth HW, Sharkey AM, Balzer DT. Novel use of the Amplatzer duct occluder to close perivalvar leak involving a prosthetic mitral valve. Catheter Cardiovasc Interv. 2004 Apr;61(4):548-551.
- Arora R, Trehan V, Rangasetty UM, Mukhopadhyay S, Thakur AK, Kalra GS. Transcatheter closure of ruptured sinus of valsalva aneurysm. J Interv Cardiol. 2004 Feb;17(1):53-58.
- Hernández-García JM, Alonso-Briales JH, Jiménez-Navarro MF, Cabrera-Bueno F, González-Cocina E, Such-Martínez M. Transcatheter closure of aorto-left atrial fistula using an Amplatzer device [in Spanish]. Rev Esp Cardiol. 2005 Sep;58(9):1121-1123.
__________________________________________
From the 1University Hospital Birmingham NHS Foundation Trust, Birmingham, United Kingdom and 2City Hospital NHS Trust, Birmingham, United Kingdom.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr DeGiovanni is on the Speaker’s Bureau for AGA Medical. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted June 21, 2011, provisional acceptance given July 18, 2011, final version accepted July 27, 2011.
Address for correspondence: Dr Yogesh Raja, Dept of Cardiology, University Hospital of Birmingham NHS Foundation Trust, Edgbaston Birmingham B15 2AT, United Kingdom. Email: dryogeshraja@gmail.com












