ADVERTISEMENT
HistoSonics Receives FDA "Breakthrough Device Designation" for Novel Sonic Beam Therapy
Breakthrough status for new histotripsy targeted liver therapy will help provide timely access to non-invasive liver treatment
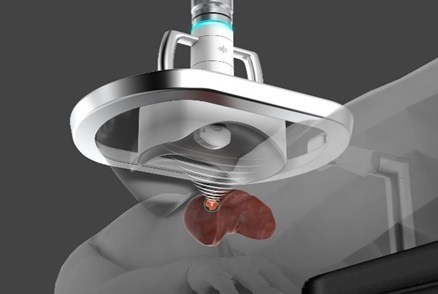
MINNEAPOLIS, Oct. 18, 2021 /PRNewswire/ -- HistoSonics, (www.histosonics.com), the developer and manufacturer of a non-invasive platform and novel sonic beam therapy called histotripsy, announced today that the U.S. Food and Drug Administration (FDA) has granted the company Breakthrough Device Designation for its new therapy platform. Histotripsy of the liver provides clinicians the first automated external beam therapy using acoustic energy to mechanically destroy and liquefy tissue in the liver without incisions, ionizing radiation or heat.
"The Breakthrough Device Designation is a significant milestone for our company and validates our belief that our platform offers significant advantages over existing approved or cleared alternatives, per FDA requirements," said Mike Blue, President and CEO of HistoSonics. "Early and ongoing clinical results are promising and suggest that our ability to precisely destroy targeted liver tissue, completely non-invasively, and without the challenges associated with ionizing radiation or other locoregional therapies, provides advantages to patients and physicians that don't exist today, and we look forward to working with the FDA to make the technology accessible as quickly as possible."
The company believes the novel mechanism of action of their proprietary technology may offer significant advantages to patients, including equivalent treatment effect throughout the entire treatment volume resulting in precise and predictable treatment zones. Early clinical and pre-clinical results also suggest that histotripsy largely preserves critical structures such as the liver capsule, and larger vessels and ducts within or adjacent to the treated volume of tissue. Additionally, histotripsy enables the treating physicians the ability to monitor the destruction of tissue under continuous real time visualization and control, unlike any modality that exists today. The Breakthrough Designation will allow the company to engage with the FDA in a prioritized review during the regulatory market authorization process.
HistoSonics has worked cooperatively with the FDA for over 3 years in developing pre-clinical and clinical data required for regulatory market authorization and intends to continue collaborating with the Agency throughout the ongoing Investigational Device Exemption (IDE) Study, #HOPE4LIVER US, which is designed to evaluate the safety and technical efficacy of histotripsy in patients with primary and secondary liver tumors. The company plans to share US and European #HOPE4LIVER Study data and results with FDA to demonstrate the benefits of histotripsy in a broad patient population.
The HistoSonics System is investigational and is not available for sale in the United States or Europe. It is limited to investigational use in the approved IDE and European studies.
About HistoSonics
HistoSonics is a privately held medical device company developing a non-invasive platform and proprietary sonic beam therapy utilizing the science of histotripsy, a novel mechanism of action that uses focused ultrasound to mechanically destroy and liquify unwanted tissue and tumors. The company is currently focused on the continued development of its Edison™ Platform, global clinical studies, and new strategic projects including future clinical applications and platforms. HistoSonics has offices in Ann Arbor, Michigan and Minneapolis, Minnesota.
For more information please visit: www.histosonics.com/
SOURCE HistoSonics, Inc.