A Novel Technique for Microsurgery on Calcified Arteries: Venous Interposition Grafting
Introduction
The indications for free tissue transfer have expanded since the advent of microsurgery over the last 50 years.1 However, microsurgery on vessels containing calcified plaque presents several unique technical challenges. As calcified vessels thicken, they become stiff and difficult to handle during surgery. Needle and anastomotic holes may remain patent, leading to leaks as elasticity is lost. Plaque formation may lead to intimal delamination by the action of the needle being driven through the vessel wall.2 Several simple solutions can facilitate success when performing micro-anastomoses between 2 calcified vessels.
Intimal Delamination
The intimal and medial layers of the arterial wall can become calcified and inelastic with advanced age when loss of elastin and plaque deposition are abetted by hyperlipidemia, diabetes, and renal insufficiency.3 The fragile intima of vasculopathic vessels easily delaminates. Intimal delamination can be addressed by passing a needle from the luminal side to the adventitial side of the artery, effectively tacking the intima to the surrounding media and adventitia in the wall of the artery.1 Microsurgical suture is generally single armed, making it necessary to pass the suture from adventitia to the lumen on one vessel end and subsequently from the lumen to adventitia on the other vessel being anastomosed. The first half of this process can push the intima away from the media, creating an area of delamination, and potential nidus for thrombosis.
Interposition vein grafting for calcified vessel anastomoses can optimize the problem of intimal delamination by suturing from tunica externa to the lumen of the vein graft and the lumen to adventitia of the calcified artery. The same process can be undertaken at the other end of the graft, tacking the arterial intima up and preventing delamination.
Stiff Vessel Handling Characteristics
Fragmentation of elastin and calcium deposits in vessel walls can also lead to poor handling quality when mobilizing vessels for anastomosis. Acute and chronic edema can further thicken vessel walls and encase the vascular tree in dense fibrotic tissue. These factors can both limit the available length of vessel that can be safely exposed and decrease the mobility of the vessel that is dissected free.1 Stiff handling characteristics make it more difficult to visualize the lumen after one or two sutures have been placed. Limited mobility can make the ergonomics of the anastomosis more challenging.
Anastomosing two calcified vessels directly together can place two noncompliant surfaces in contact, creating a poor seal and subsequent leakage. Commonly used anticoagulants, such as aspirin and heparin, may exacerbate hematoma formation in this patient population.4,5
Interposition vein grafting can add flexibility to the anastomosis that serves to improve the ergonomics and visualization of the intima.1 Because veins are less affected by calcification than arteries, the supple vein can be more easily sewn and allows one end of the anastomosis to be performed with improved degrees of freedom, followed by the second end of the anastomosis with equally improved handling and visualization.
Leaking Suture Holes
Calcified plaque is difficult to puncture with standard microsurgery needles (eg, BV 130-5) and dulls the needle tip quickly. Use of cutting needles can lead to micro-tearing and leaks when sewing these delicate vessels. Leaking at suture holes can be difficult to solve, as further suture placement can lead to more leaking. Tapered needles with a cutting tip may help puncture the plaque while avoiding propagation of the puncture into a laceration (eg, Sharpoint, Wyomissing, PA).
Methodology/Case Presentation
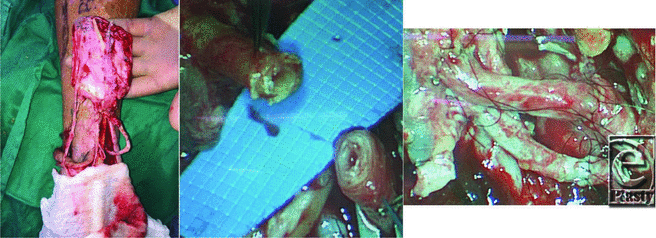
A 64-year-old previously ambulatory diabetic man with a history of tobacco use presented with bilateral stage IV pressure ulcers of the heels after an incapacitating illness (Figure 1). The wounds were grossly debrided, and bone biopsy samples were taken to guide antibiotic management for osteomyelitis. Ankle brachial indices were less than 0.4 for both extremities, indicating critical limb ischemia. Angiography showed multilevel arterial disease for the left lower extremity and a short-segment vascular occlusion on the right that was amenable to and subsequently underwent successful balloon angioplasty (Figure 2). The operative plan devised was to perform a left below-knee amputation and use spare parts from the amputated left leg in the form of a dorsalis pedis free flap to cover the right heel (Figure 3a). Both the donor and recipient arteries were significantly calcified, with delaminating intimal plaque and stiff, edematous walls (Figure 3b). Initial end-to-end anastomosis between the flap's anterior tibial artery and the recipient right posterior tibial artery was challenging due to poor mobility of the fibrotic recipient artery and intimal delamination. There was also significant leaking from the anastomotic edge and suture holes. To solve these problems, an interposition vein graft was used from the left greater saphenous vein, creating a more favorable anastomosis. This was particularly helpful at the stiff recipient right posterior tibial artery, which was anastomosed first. To secure the plaque to the underlying arterial wall, all sutures were placed from tunica externa to the lumen on the vein and the lumen to adventitia on the recipient posterior tibial artery as well as for the free flap's anterior tibial artery (Figure 3c). The final flap position after inset and anastomosis is shown in Figure 4a.
 |
|
Figure 1. Bilateral chronic heel pressure ulcers with calcaneal osteomyelitis.
|
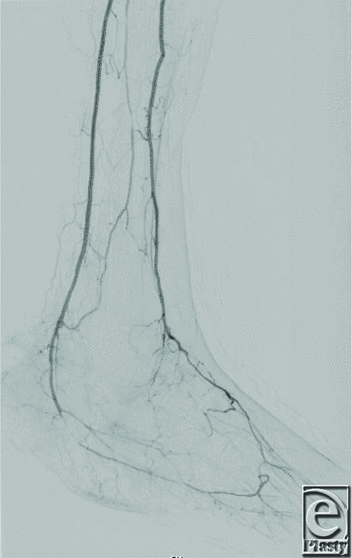 |
| Figure 2. Right lower extremity angiography following angioplasty of the posterior tibial artery demonstrating restored inflow. |
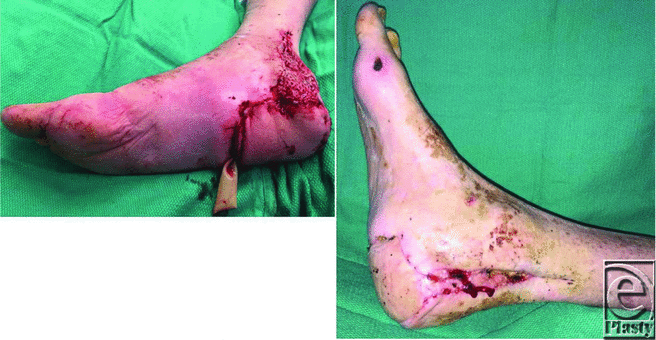 |
| Figure 4. (a) Right heel soft tissue reconstruction with the dorsalis pedis spare part flap from the left foot. (b) Right foot at 6 weeks after surgery. |
Discussion
Heel ulcers in patients with chronic vascular disease often herald amputations due to poor reconstructive outcomes.6 Microsurgical options in situations of severe arterial calcification are often complicated by fibrotic peripheral vasculature with anastomotic leakage and intimal delamination.
Challenges related to the management of advanced peripheral arterial disease have become more prevalent in the Western society as the incidence of dysmetabolic disease rises.3 While the experience in vascular surgery for macrovascular disease in atherosclerotic vessels continues to advance for reestablishing inflow, that for surgery on microvascular diseased vessels is rarely reported in the literature.1,6,7 In addition, prior reports have described vein grafting in microsurgical anastomosis to solve the problem of inadequate pedicle length rather than to facilitate the anastomosis between calcified vessels.1,8 Mücke and others9 studied the efficacy of performing atherectomy in microvessels in a rat injury model and concluded that the intimal injury produced leads to a high risk of thrombosis after completing the anastomosis. Although this procedure may provide a more local solution to dealing with calcified macrovasculature, atherectomy is not usually a viable option for optimizing calcified vessels before micro-anastomosis due to its thrombogenic potential.
Finally, while venous interposition is a useful and versatile technique in difficult reconstructive situations, it can in itself be associated with increased rates of pedicle thrombosis due to flow passing through two anastomoses for each vessel.10 This limitation must be considered before resorting to this surgical option and should only be used when traditional anastomotic techniques either have failed or are likely to perform poorly.
Summary and Implications
Interposition vein grafting can improve the ergonomics of microsurgical anastomosis in calcified vessels. The compliant nature of vein grafts can create an improved seal when anastomosed to the more rigid calcified artery. It is our hope that this simple technique could provide a reconstructable solution for this difficult clinical problem.
Acknowledgments
Authors: Ray Christopher Hosein, MDa, Andrei Odobescu, MDb, and Isak A. Goodwin, MDa
Affiliations: aDivision of Plastic & Reconstructive Surgery, University of Utah Health, Salt Lake City, UT; bDivision of Plastic & Reconstructive Surgery, University of Iowa Hospitals and Clinics, Iowa City, IA
Correspondence: Isak.Goodwin@hsc.utah.edu
Disclosures: The authors disclose no financial or other conflicts of interest
References
1. Chen HC, Coskunfirat OK, Ozkan O, et al. Guidelines for the optimization of microsurgery in atherosclerotic patients. Microsurgery. 2006;26(5):356-62.
2. Cigna E, Lo Torto F, Parisi P, Felli A, Ribuffo D. Management of microanastomosis in patients affected by vessel diseases. Eur Rev Med Pharmacol Sci. 2014;18(22):3399-405.
3. Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review & perspective. Hypertension. 2010;55(3):579-92.
4. Ashjian P, Chen CM, Pusic A, Disa JJ, Cordeiro PG, Mehrara BJ. The effect of postoperative anticoagulation on microvascular thrombosis. Ann Plast Surg. 2007;59(1):36-9, discussion 39-40.
5. Fitzgerald O'Connor EJ, Vesely M, Holt PJ, Jones KG, Thompson MM, Hinchliffe RJ. A systematic review of free tissue transfer in the management of non-traumatic lower extremity wounds in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41(3):391-9.
6. Kallio M, Vikatmaa P, Kantonen I, Lepäntalo M, Venermo M, Tukiainen E. Strategies for free flap transfer and revascularisation with long-term outcome in the treatment of large diabetic foot lesions. Eur J Vasc Endovasc Surg. 2015;50(2):223-30.
7. Banis JC Jr, JD Richardson, Derr JW Jr, Acland RD. Microsurgical adjuncts in salvage of the ischemic and diabetic lower extremity [review]. Clin Plast Surg. 1992;19(4):881-93.
8. Suh HS, Oh TS, Lee HS, et al. A new approach for reconstruction of diabetic foot wounds using the angiosome and supermicrosurgery concept. Plast Reconstr Surg. 2016;138(4):702e-9e.
9. Mücke T, Wolff C, von Düring M, Mitchell DA, Ritschl LM, Fichter AM. Form and size matter: increased risk of thrombosis in microvessels with surgically created endothelial lesions. J Reconstr Microsurg. 2017;33(1):40-4.
10. Nelson JA, Fischer JP, Grover R, et al. Vein grafting your way out of trouble: examining the utility and efficacy of vein grafts in microsurgery. J Plast Reconstr Aesthet Surg. 2015;68(6):830-6.