LETTER TO THE EDITOR Abnormal Responses of Keloid Tissue to Wounding Identified Using In Vitro Model System
| LETTER TO THE EDITOR | |
| Abnormal Responses of Keloid Tissue to Wounding Identified Using In Vitro Model System | |
| ,a,b ,a ,a ,a b,a | |
aThe Research Department, The Shriners Hospitals for Children—Cincinnati; and bDepartment of Surgery, The University of Cincinnati, College of Medicine, Cincinnati, OH | |
Correspondence: dsupp@shrinenet.org |
|
|
Dear Sir,
Keloids are thick, raised scars that represent an extreme form of abnormal scarring. Unlike normal scars, keloids extend beyond the original wound margin and rarely regress; instead, they tend to proliferate indefinitely.1-4 These bulky scars can significantly impair function due to itching, pain, and decreased range of motion5 and can negatively impact psychosocial well-being and overall quality of life.5-7 Although many different therapeutic modalities exist, keloids are extremely resistant to treatment and have a high rate of recurrence.1,2,8-10 Development of effective, targeted interventions has been limited due to an incomplete understanding of the pathophysiology of keloid scarring. Furthermore, because keloid scarring is only found in humans, there are no animal models of keloid scarring, which has hindered the evaluation of novel therapies. Ethical considerations preclude wound healing studies in patients susceptible to keloid scarring. Therefore, in the absence of suitable animal models, organotypic models represent a feasible alternative for investigation of wounding in keloid tissue. In the current study, we investigated engineered skin substitutes (ESS) composed of keratinocytes, fibroblasts, and collagen-glycosaminoglycan biopolymers, as an in vitro organotypic keloid model to analyze changes in gene expression in response to wounding.
Primary human fibroblasts and keratinocytes were isolated and cultured11,12 from excised keloid scar or normal skin, collected with University of Cincinnati Institutional Review Board approval. ESS were prepared by sequential inoculation of fibroblasts and keratinocytes onto approximately 40 cm2 collagen-glycosaminoglycan biopolymer sponges.11,13,14 After 7 days of culture at the air-liquid interface, ESS were cut in half, and one piece of each was wounded using a skin graft mesher; approximation of the cut edges permitted healing of the wounded ESS to occur.
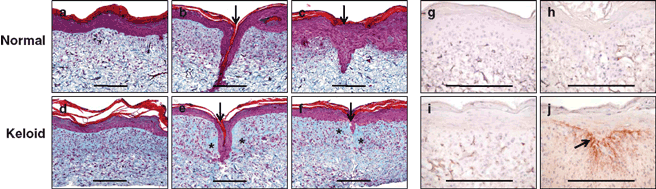
Histological analysis of sections of normal and keloid ESS 14 days after keratinocyte inoculation demonstrated a well-stratified epidermal layer and a dermal component populated with fibroblasts (Figs 1a-1f). Seven days after wounding, migration of keratinocytes into the wound edge occurred to varying degrees in both normal and wounded ESS. In keloid ESS, newly synthesized collagen was visible in the area adjacent to the wound, as evidenced qualitatively in trichrome-stained sections. This was not observed in the normal ESS after wounding.
Immunohistochemistry was performed to localize periostin, a matricellular protein that has been shown to be expressed at higher levels in keloid fibroblasts than in normal fibroblasts.15 In contrast to normal ESS or nonwounded keloid ESS, high levels of periostin were detected in wounded keloid ESS and were localized to the upper dermis and dermal-epidermal junction in the region of the healing wound (Figs 1g and 1h).
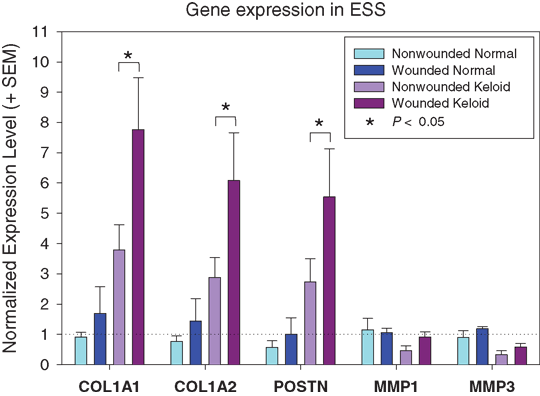
Quantitative real-time PCR16,17 was used to analyze expression of genes previously implicated in keloid scarring: type 1 collagen pro-alpha 1 and 2 chain genes (COL1A1 and COL1A2); matrix metalloproteinases 1 and 3 (MMP1 and MMP3); and periostin (POSTN)15,18-22 (Fig 2). Expression levels for COL1A1, COL1A2, and POSTN were higher in nonwounded keloid ESS than in nonwounded normal ESS. Upon in vitro wounding, expression of these genes was slightly elevated in normal ESS but the differences were not statistically significant. In contrast, COL1A1, COL1A2, and POSTN expression levels were significantly increased in wounded keloid ESS. MMP1 and MMP3 were expressed at lower levels in keloid ESS compared with normal ESS and were not significantly increased after wounding.
The results obtained using ESS as an in vitro organotypic model are consistent with the hypothesis that an imbalance of extracellular matrix (ECM) production and degradation after wounding leads to keloid scarring. In our organotypic model, keloid cells respond to injury with an exaggerated increase in ECM production. The expression of genes involved in ECM breakdown is insufficient to counteract this significant increase in matrix production. We propose that this in vitro model will provide a useful system for further identification of abnormal interactions between keloid fibroblasts, keratinocytes, and ECM, and screening of novel therapies.
1. Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4(4):235-43. |
2. English RS, Shenefelt PD. Keloids and hypertrophic scars. Dermatol Surg. 1999;25:631-8. |
3. Datubo-Brown DD. Keloids: a review of the literature. Br J Plast Surg. 1990;43:70-7. |
4. Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18:396-402. |
5. Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433-8. |
6. Van Loey NEE, Van Son MJM. Psychopathology and psychological problems in patients with burn scars. Am J Clin Dermatol. 2003;4(4):245-72. |
7. Olaitan PB. Keloids: assessment of effects and psychosocial-impacts on subjects in a black African population. Indian J Dermatol Venereol Leprol. 2009;75(4):368-72. |
8. Murray JC. Keloids and hypertrophic scars. Clin Dermatol. 1994;12(1):27-37. |
9. Davidson S, Aziz N, Rashid RM, Khachemoune A. A primary care perspective on keloids. Medscape J Med. 2009;11(1):18. |
10. Gupta S, Sharma VK. Standard guidelines of care: keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol. 2011;77(1):94-100. |
11. Boyce ST. Methods for serum-free culture of keratinocytes and transplantation of collagen-GAG based composite grafts. In: Morgan JR, Yarmush M, eds. Tissue Engineering Methods and Protocols. Totowa, NJ: Humana Press; 1998:365-89. |
12. Boyce ST, Supp AP, Swope VB, Warden GD. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane formation in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol. 2002;118:565-72. |
13. Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res. 1988;22:939-57. |
14. Smiley AK, Klingenberg JM, Aronow BJ, et al. Microarray analysis of gene expression in cultured skin substitutes compared with native human skin. J Invest Dermatol. 2005;125:1286-301. |
15. Zhou HM, Wang J, Elliott C, et al. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal. 2010;4(2):99-107. |
16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402-8. |
17. McFarland KL, Klingenberg JM, Boyce ST, Supp DM. Expression of genes encoding antimicrobial proteins and members of the toll-like receptor/nuclear factor-kappaB pathways in engineered human skin. Wound Repair Regen. 2008;16(4):534-41. |
18. Smith JC, Boone BE, Opalenik SR, Williams SM, Russell SB. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol. 2007;128(5):1298-310. |
19. Tsou R, Cole JK, Nathens AB, et al. Analysis of hypertrophic and normal scar gene expression with cDNA microarrays. J Burn Care Rehabil. 2000;21(6):541-50. |
20. Chen W, Fu X, Sun X, et al. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003; 113(2):208-16. |
21. Wu J, Ma B, Yi S, et al. Gene expression of early hypertrophic scar tissue screened by means of cDNA microarrays. J Trauma. 2004;57(6):1276-86. |
22. McFarland KL, Glaser K, Hahn JM, Boyce ST, Supp DM. Culture medium and cell density impact gene expression in normal skin and abnormal scar-derived fibroblasts. J Burn Care Res. 2011;32(4):498-508. |
| JOURNAL INFORMATION | ARTICLE INFORMATION |
| Journal ID: ePlasty | Volume: 12 |
| ISSN: 1937-5719 | E-location ID: e19 |
| Publisher: Open Science Company, LLC | Published: April 12, 2012 |