Influence of Receptor Antagonists, Local Anesthetics, and Denervation on Microcirculation
| Influence of Receptor Antagonists, Local Anesthetics, and Denervation on Microcirculation | |
| ,a ,b ,b ,c ,b ,d b | |
aDepartment of Plastic, Reconstructive and Aesthetic Surgery, Hand Surgery, Helios-Klinikum Wuppertal, University of Witten/Herdecke, Germany; bBG-University Hospital Bergmannsheil, Department of Plastic and Hand Surgery, Burn Center, Ruhr-University Bochum, Germany; cPlastic Surgery/Migraine Surgery, DRK-Hospital Berlin, Germany; and dBG-Hospital Ludwigshafen, Department of Hand-, Plastic- and Reconstructive Surgery, Burn Center, University of Heidelberg, Germany | |
Correspondence: olegoertz@gmx.de |
|
Objective: Impaired microcirculation is one of the most important factors in delayed wound healing. The aim of the study was to investigate the influence of chemical and surgical interruption of sympathetic nerve fibers and α- and β-receptors blockers on muscular microcirculation. Methods: The experiment was performed on a standardized cremaster muscle model of male Wistar rats (n=51). Microcirculation was recorded via transillumination microscopy on each of the 4 test groups and in a control group before and after their respective treatments with one of the following: topical application of bupivacaine, metoprolol, phentolamine, or surgical denervation. The arteriolar diameter and functional capillary density (FCD) as parameter for tissue perfusion were assessed. Results: The α-blocker phentolamine was the only agent that caused a significant dilation of the arteriolar diameter (76.6 ± 6.9 vs 100.0 ± 12.0 µm). However, like bupivacaine, metoprolol, and the surgical sympathectomy, it did not improve FCD as a parameter for tissue perfusion. The strongest vasoconstriction (35.9 ± 4.3 vs 28.6 ± 4.0) and impairment of the FCD (10.0 ± 0.7 vs 4.1 ± 0.9) was induced by the β-blocker metoprolol. Conclusions: This study shows that phentolamine could be an agent for dilating arteriolar diameter, but it did not improve FCD. Whereas the other agents, including sympathectomy, did not alter arteriolar diameter, the β-blocker worsened both investigated parameters. Our results raise the question whether β-blockers negatively influence microcirculation. Therefore, further studies are needed to investigate the potential adverse effects of β-blockers on wound healing. |
Wound healing is a process involving complex cellular and extracellular mechanisms. It is well-known that wound healing depends mainly on tissue perfusion, and more specifically, on microcirculation. If the microcirculation is impaired, for example, by diabetes mellitus, radiation therapy, bacterial infection, or peripheral arterial occlusive disease, wound healing is impaired.1 It is therefore of the utmost importance that wound healing will not be further inhibited by any agent, and it would be desirable if topically applied agents could improve microcirculation.
In addition to metabolic influences, myogenic factors are responsible for the regulation of the vessel diameter in muscles and therefore for muscle perfusion.2 Precapillary sphincter systems control the capillary perfusion. The myogenic regulation is mediated by α- and β-receptors. The α1-receptors with their subtypes regulate primarily the first-order arterioles, whereas the α2-receptors regulate the third-order arterioles.3-6
Because many patients suffer from heart and blood pressure diseases, β-blockers are widely used. These same patients also often suffer from chronic dermal ulcers. β-blockers could be one factor in decreasing peripheral perfusion and, therefore, could impair wound healing.
There is also the question of whether the clinically used medical or surgical sympathectomy (Raynaud disease, arterial occlusive disease) alter arteriolar diameter and functional capillary density (FCD).7-10
The aim of our study was to investigate the effect of topically applied α- and β-receptor blockers, local anesthetics, and denervation on the muscular microcirculation in a rat model. An applied drug or intervention causing an increase of the arteriolar diameter and functional vessel density could be used in combination with antibacterial agents for treatment of critical wounds. Conversely, a drug causing decrease of these parameters should be avoided.
METHODS
Animals
The cremaster muscle of male Wistar rats were the subject of the study (n=51, 170-200 g; Harlan & Winkelmann, Borchen, Germany). The experiments were conducted in accordance with the National Research Council's guide for the care and use of laboratory animals.
Anesthesia
Animals were anesthetized by intraperitoneally injected sodium pentobarbital (Narcoren, 50 mg/kg body weight; Merial GmbH, Hallbergmoos, Germany). If necessary, /one third of the initial dose was injected to maintain anesthesia. The animals were placed on a heated observation platform (Effenberger, Pfaffing, Germany), rectal temperature, and surface temperature of the prepared muscle were measured throughout the whole study period by using a 2-probe thermometer (Atkins Technical Inc, Mod 39658T, Gainesville, Florida).
After shaving the throat, the left carotid artery was prepared and a probe was inserted to monitor the blood pressure continuously throughout the study (Intramedic Polyethylene Tubing, PE-50, Becton Dickson, Parippany, NY; blood pressure meter: Monitor BP-1, World Precision Instruments, Berlin). To stabilize the blood pressure, saline solution was injected through the same catheter. A tracheotomy was performed to keep the airway open using tubing (Venofix, Luer Lock 21G, 0.8 × 2.0 mm, Braun, Melsungen, Germany), while animals breathed spontaneously.
Cremaster Preparation
The model was first described by Baez et al.11 After isolating of the cremaster muscle, it was stretched using 7 loops on a specially designed observation platform (Fig 1), where the temperature was monitored.12
Test solution and study groups
The α1-receptors were blocked by the topical application of phentolamine hydrochloride (n=8; Sigma Chemicals, Seelze, Germany), which was dissolved in aqua purificata (20 mg/mL), resulting in a concentration of 5 mg/cm2 at the cremaster muscle.13,14 The β-receptors were blocked by metoprolol tartrate (n=8; 3 mg, Beloc, Astra, Wedel, Germany).
The sympathetic fibers were suppressed by topical application of bupivacaine (n=8; 0.5%, 15 mg bupivacaine hydrochloride; Carbostesin, Astra, Wedel, Germany).
The denervation (n=15) was performed at the proximal cremaster muscle, where the periarteriolar tissue, including the nerves, were dissected.15
Saline solution (n=12; Natriumchlorid, B. Braun Melsungen AG, Melsungen, Germany) was used for control.
A volume of 3 mL of the solutions was applied to the surface of the muscle for 30 minutes and covered by a piece of self-adherent polyurethane dressing (OpSite? Dressing Film, Smith and Nephew Wound Management, Largo, Florida) to prevent the muscle for drying out and to keep the solution on the muscle. After 30 minutes, the solutions were rinsed out with saline solution.
Exclusion criteria
If arterial blood pressure dropped below 80 mm Hg or exceeded 120 mm Hg even after intervention or if rectal body temperature (34.5°C-37.5°C) and temperature of the cremaster muscle surface (31.0°C-34.0°C) departed from the determined values, the animals were excluded from the study.
Microscopy and recordings
For the transillumination microscopy (Axiotech vario, Carl Zeiss, Oberkochen, Germany) and recording, a charge-coupled video camera was used (Model Nr MC-3309, AVT-Horn, Aalen, Germany) and the recordings stored on super VHS videotapes (Panasonic AG-7350). Microscopic observations were performed before application of agents as well as 60 and 120 minutes after. Altogether 9 visual fields with a dimension of 0.55 to 0.40 mm were investigated.
We used a 10-fold water immersion objective (Achroplan 4x/20x, Zeiss), resulting in a total magnification of about 100-fold. Photographs were taken to ensure accurate relocation of the investigated areas throughout the study.
The diameter of the first (A1)-, second (A2)-, and third-order (A3) arteries in µm, as well as the perfused capillaries per area (0.22 mm2), was measured. After concluding the experiments, the animals were euthanized by an overdose of pentobarbital.
Statistics
The commercially available computer program SPSS version 18 (SPSS GmbH, Munich, Germany) was used for statistical analysis of the data. The data collection of the recordings was blinded; the researcher did not know the interval of time between recordings or the agent being tested. Out of the single data, the mean value, the standard error, and the standard error of the mean of each animal were calculated. To compare the different values with each other, a variance analysis for repeated measurements was used. The mean value of the significant data was compared with the t test for paired samples. A P < .05 was considered statistically significant.
RESULTS
The cremaster model in rats allows for good-quality recordings of arteriolar diameter and capillary function (Fig 1). In none of the animals, the core and muscle temperature or blood pressure exceeded or dropped below the determined values (Table 1).
| Table 1. Weight, Rectal Temperature, Temperature of the Cremaster Muscle, and Blood Pressure of the Five Groups* | |||||
| Parameter | Control | Denervation | Bupivacaine | Metoprolol | Phentolamine |
| Animal weight, g | 182.7 ± 3.0 | 185.3 ± 1.6 | 186.9 ± 1.9 | 187.4 ± 2.4 | 186.8 ± 1.8 |
| Rectal temperature, 0 min, °C | 35.6 ± 0.2 | 35.7 ± 0.2 | 35.7 ± 0.2 | 36.0 ± 0.2 | 35.9 ± 0.2 |
| Rectal temperature, 60 min, °C | 35.6 ± 0.2 | 35.4 ± 0.3 | 35.4 ± 0.4 | 35.8 ± 0.2 | 35.8 ± 0.2 |
| Muscle temperature, 0 min, °C | 32.2 ± 0.2 | 32.9 ± 0.2 | 33.1 ± 0.2 | 32.9 ± 0.2 | 32.8 ± 0.3 |
| Muscle temperature, 60 min, °C | 32.3 ± 0.3 | 32.4 ± 0.3 | 32.6 ± 0.3 | 32.6 ± 0.2 | 32.7 ± 0.2 |
| Blood pressure,† 0 min | 106.8 ± 1.6 | 95.2 ± 6.6 | 95.5 ± 8.3 | 106.3 ± 1.8 | 102.8 ± 2.7 |
| Blood pressure,† 60 min | 99.8 ± 2.2 | 95.1 ± 1.7 | 96.3 ± 1.8 | 95.5 ± 1.8 | 98.3 ± 2.2 |
| *Data is given in mean and standard error of mean; 0 = baseline value. | |||||
| †Blood pressure measured within the carotid arteries. | |||||
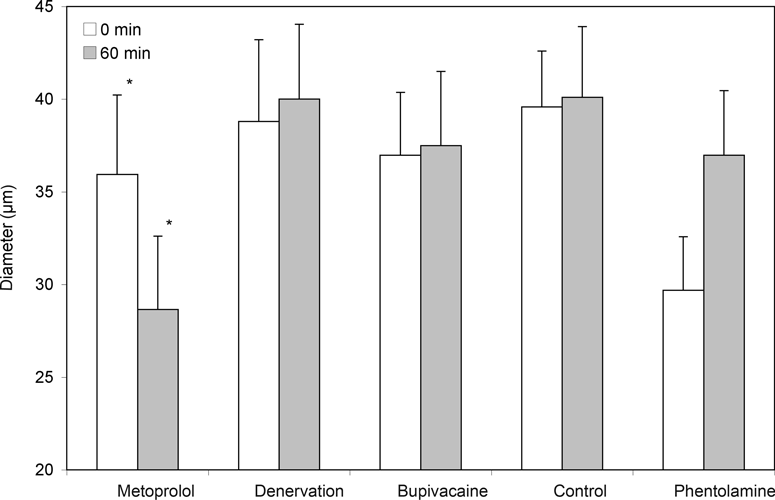
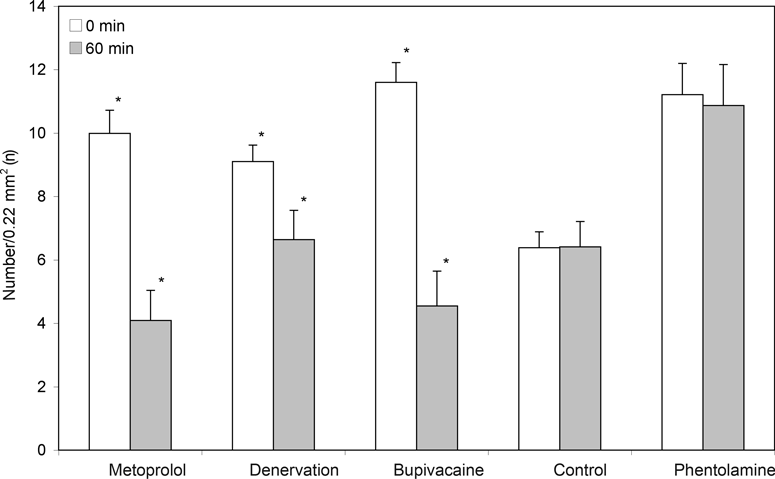
For the control group, no alterations of arteriolar diameter or FCD could be found at any of the measured points in time (Figs 2-5, Table 2).
The denervation of the sympathetic fibers caused a vasodilation, which occurred immediately after surgical sectioning and lasted for 30 minutes. After 60 minutes, we saw a decrease of the arteriolar diameter in A1- and A2-arterioles but no alterations in A3-arterioles (Table 2, Fig 2). The FCD decreased significantly (Table 2, Fig 3).The local anesthetic bupivacaine also caused a decrease of A1- and A2-arterioles and showed no influence on A3-arterioles.
| Table 2. Diameter and Functional Capillary Density of the 5 Groups* | ||||||
| Parameter | Time | Control | Denervation | Bupivacaine | Metoprolol | Phentolamine |
| A1-arterioles [µm] | 0 | 80.73 ± 4.25 | 85.16 ± 3.21 | 90.10 ± 4.03 | 79.17 ± 3.38 | 76.56 ± 6.89 |
| 60 | 86.98 ± 5.98 | 77.87 ± 4.68 | 80.73 ± 7.32 | 72.92 ± 6.27 | 100.00 ± 12.02 | |
| A2-arterioles [µm] | 0 | 60.94 ± 3.28 | 67.66 ± 5.08 | 66.15 ± 3.56 | 64.58 ± 7.74 | 51.04 ± 4.13 |
| 60 | 59.90 ± 4.52 | 64.58 ± 5.20 | 59.90 ± 5.13 | 54.17 ± 7.93 | 64.58 ± 8.01 | |
| A3-arterioles [µm] | 0 | 39.58 ± 3.01 | 38.80 ± 4.41 | 36.98 ± 3.39 | 35.94 ± 4.29 | 29.69 ± 2.89 |
| 60 | 40.10 ± 3.80 | 40.00 ± 4.04 | 37.50 ± 4.00 | 28.65 ± 3.96† | 36.98 ± 3.48 | |
| FCD [n/0.22 mm2] | 0 | 6.38 ± 0.51 | 9.11 ± 0.52 | 11.60 ± 0.63 | 9.99 ± 0.73 | 11.21 ± 0.98 |
| 60 | 6.41 ± 0.80 | 6.64 ± 0.93† | 4.55 ± 1.10† | 4.09 ± 0.95† | 10.87 ± 1.29 | |
| *Data is given in mean and SEM; FCD indicates functional capillary density. | ||||||
| † P < .05 versus baseline value; | ||||||
The blockade of the β-receptors by metoprolol caused a decrease in arteriolar diameter in all arteriolar orders and caused the last-order arterioles to differ significantly from the baseline value. In addition, a significant decrease of the FCD could be found.
After the blockade of α1-receptors by phentolamine, an increase in arteriolar diameter was noticed in A1, A2, and A3-arterioles, but the values did not differ significantly from the baseline value. The FCD was not influenced by phentolamine.
The blockade of β-receptors by metoprolol led to a decrease in arteriolar diameter, with the values of the third-order arterioles differing significantly (P < .05) from those of the control animals (Table 2, Fig 2). In addition, a significant decrease of the FCD, as compared to the baseline value, could be found (Fig 3).
Both surgical denervation and chemically blocking the postganglionare sympathic fibers by bupivacaine caused a change in arteriolar diameter and caused a significant decrease of the FCD, as compared to base values (Table 2, Figs 2 and 3).
DISCUSSION
The isolated cremaster muscle model in rats allows for the direct visualization of the muscular microcirculation and, therefore, for direct investigations of the impact of topically applied agents.11 No alteration of the blood flow, the critical aspect of the model, could be found after preparation of the cremaster muscle in the past.15
After denervation of the sympathetic fibers of the cremaster muscle, the influence of the central nerve system on the vasomotion is interrupted.16 Directly after the surgical sectioning of the fibers, we observed a vasodilation lasting 30 minutes. These observations confirm the results of Chen et al.17 After 1 hour, the arteriolar diameter was unaffected, but the FCD—as parameter for tissue perfusion—decreased significantly. An increase of the FCD was observed 14 days after sectioning by Bentzer et al.18 Long-term follow-up studies have shown that the FCD decreases after denervation to 10% of its initial value, which could be interpreted as an adequate value for the reduced physiological requirements.19 In contrast to our results, in rabbits, an increase of the cutaneous arteriolar diameter after periarterial sympathectomy was found.20
Bupivacaine, a local anesthetic of the amino amide type, acts mainly by binding itself to the intracellular portion of sodium channels and blocking sodium influx into nerve cells, thereby blocking depolarization, as well. In our study, the A1- and A2-arterioles showed a slight vasoconstriction, the A3-arterioles were unaffected, and the FCD diminished significantly. This could be the result of the low level of bupivacaine concentration. Low bupivacaine concentration causes vasoconstriction, while high concentration causes vasodilation.21 Another reason could be the reduction of the vasodilative potency of the endothelium by the local anesthesia.22
The high dose of metoprolol was chosen because at that level, it blocks either the β1- or β2-receptors. The observed constriction of all arteriolar orders is caused by the blockade of the β-receptors, which have adrenergically mediated vasodilative effects. As a result of these findings, we believe that further studies are necessary. In the meantime, the use of β-receptor blockers in patients with critically perfused wounds or within microsurgical interventions should be critically evaluated, because the same effects have been found in humans.7
After blockade of α-receptors by phentolamine, adrenergically mediated influences should be minor, and we expected a stronger increase in arteriolar diameter than we found. This effect could have been caused by the blockade of all α-receptors, including the α2D-receptors, which normally cause vasodilation mediated by nitric oxide.13 Another study found that blockade of α-receptors caused vasodilation in humans as well.7 The different sensitivities of the α1- and α2-receptors play a minor role, because in the applied concentrations, the blockade could be assumed to be complete.23,24 This could explain the lack of influence on the FCD. In contrast to our results, a study on the perfusion of the ileum in rabbits showed an impairment of blood flow after the systemic administration of an α adrenoceptor blocker.25 This could be because the systemic administration of the blocker induces hypotension in the animal. We tried to compensate for possible systemic reactions by infusing physiological saline into the animal.
Our results show that, of the 5 groups, only the α-receptor blocker phentolamine causes vasodilation after 1 hour. A surgical or chemical interruption of the sympathetic fibers caused no increase in vasodilation. Rather, it reduced the FCD in a similar manner to topically applied metoprolol, the agent that caused the greatest decrease in diameter and the greatest impairment of FCD. These results raise the question of whether or not these frequently performed sympathetic blockades really improve microcirculation.7
Many patients who undergo surgical or even microsurgical intervention are on antihypertensive medications, including β-blockers, during the perioperative phase. Our results raise the question of whether β-blockers negatively influence microcirculation. Therefore, we believe that further studies are needed to investigate this potential negative side effect.
Acknowledgment
The authors thank the Alma und Heinrich Vogelsang Foundation, Bochum, Germany, and the scientific committee, Bergmannsheil, Bochum, Germany (both foundations had no content-related involvement), for their financial support.
1. Suh DY, Hunt TK. Time line of wound healing. Clin Podiatr Med Surg1998;15:1-9. |
2. Koo A, Liang IY. Parasympathetic cholinergic vasodilator mechanism in the terminal liver microcirculation in rats. Q J Exp Physiol Cogn Med Sci1979;64:149-59. |
3. Lundvall J, Hillman J. Noradrenaline evoked beta adrenergic dilatation of precapillary sphincters in skeletal muscle. Acta Physiol Scand. 1978;102:126-8. |
4. Lundvall J. Tissue hyperosmolality as a mediator of vasodilatation and transcapillary fluid flux in exercising skeletal muscle. Acta Physiol Scand Suppl. 1972;379:1-142. |
5. Hillman J, Lundvall J. Classification of beta-adrenoceptors in the microcirculation of skeletal muscle. Acta Physiol Scand. 1981;113:67-71. |
6. Koo A. Vasodilator effect of terbutaline: in vivo evidence for the existence of beta 2-adrenoceptors in the microcirculation of rats, hamsters, and guinea pigs. J Cardiovasc Pharmacol. 1984;6:897-901. |
7. Johansson K, Eriksson M, Wahlqvist I, von zur Muhlen B, Lind L. Effects of blockade of alpha- and beta-adrenoceptors and neuropeptide Y(1) receptors, as well as brachial plexus blockade, on endothelium-dependent vasodilation in the human forearm. Clin Exp Pharmacol Physiol. 2002;29:603-7. |
8. Backman SB. Regional anesthesia: sympathectomy-mediated vasodilation. Can J Anaesth. 2009;56:702-3; author reply 703. |
9. Maga P, Kuzdzal J, Nizankowski R, Szczeklik A, Sladek K. Long-term effects of thoracic sympathectomy on microcirculation in the hands of patients with primary Raynaud disease. J Thorac Cardiovasc Surg. 2007;133:1428-33. |
10. Della Giovampaola C, Conte M, Caldarelli C, et al. Retroperitoneoscopic lumbar sympathectomy for nonreconstructible arterial occlusive disease. Minerva Chir. 2006;61:409-15. |
11. Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384-394. |
12. Siemionow M, Wang WZ, Anderson G, Firrell J. Leukocyte-endothelial interaction and capillary perfusion in ischemia/reperfusion of the rat cremaster muscle. Microcirc Endothelium Lymphatics. 1991;7:183-97. |
13. Leech CJ, Faber JE. Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am J Physiol. 1996;270:H710-22. |
14. Sakanashi M, Hiraki I. Effects of vasodilators on microcirculation of the rat cremaster muscle: a microscopic method for screening drugs. Jpn J Pharmacol. 1979;29:125-31. |
15. Lohman R, Siemionow M, Lister G. Advantages of sharp adventitial dissection for microvascular anastomoses. Ann Plast Surg. 1998;40:577-85. |
16. Fleming BP, Barron KW, Howes TW, Smith JK. Response of the microcirculation in rat cremaster muscle to peripheral and central sympathetic stimulation. Circ Res. 1987;61:1126-31. |
17. Chen LE, Seaber AV, Bossen E, Urbaniak JR. The effect of acute denervation on the microcirculation of skeletal muscle: rat cremaster model. J Orthop Res. 1991;9:266-74. |
18. Bentzer P, Nielsen N, Arner M, et al Supersensitivity in rat micro-arteries after short-term denervation. Acta Physiol Scand. 1997;161:125-33. |
19. Borisov AB, Huang SK Carlson BM. Remodeling of the vascular bed and progressive loss of capillaries in denervated skeletal muscle. Anat Rec. 2000;258:292-304. |
20. Pollock DC, Li Z, Rosencrance E, Krome J, Koman LA Smith TL. Acute effects of periarterial sympathectomy on the cutaneous microcirculation. J Orthop Res. 1997;15:408-13. |
21. Johns RA, Seyde WC, DiFazio CA Longnecker DE. Dose-dependent effects of bupivacaine on rat muscle arterioles. Anesthesiology. 1986;65:186-91. |
22. Johns RA. Local anesthetics inhibit endothelium-dependent vasodilation. Anesthesiology. 1989;70:805-11. |
23. Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174-84. |
24. Dohler JR, Hughes SP. [Morphologic effect of adrenaline and insulin on bone capillaries after receptor blockade. Functional transmission electron microscopy analysis]. Langenbecks Arch Chir. 1997;382:164-6. |
25. Lehmann C, Lewerenz A, Kreyer I Luther B. Effects of alpha 1-adrenoceptor blocker prazosin on microcirculation in terminal rabbit ileum. Acta Chir Hung. 1989;30:273-80. |
| JOURNAL INFORMATION | ARTICLE INFORMATION |
| Journal ID: ePlasty | Volume: 11 |
| ISSN: 1937-5719 | E-location ID: e2 |
| Publisher: Open Science Company, LLC | Published: January 20, 2011 |