A Contractile Network of Interstitial Cells of Cajal in the Supratarsal Mueller’s Smooth Muscle Fibers With Sparse Sympathetic Innervation
| A Contractile Network of Interstitial Cells of Cajal in the Supratarsal Mueller's Smooth Muscle Fibers With Sparse Sympathetic Innervation | |
| ,a ,a ,a ,a b | |
Departments of aPlastic and Reconstructive Surgery and bAnatomy, Shinshu University School of Medicine, Matsumoto, Japan. | |
Correspondence: kmatsuo@shinshu-u.ac.jp |
|
Background: We previously reported that the supratarsal Mueller's muscle is innervated by both sympathetic efferent fibers and trigeminal proprioceptive afferent fibers, which function as mechanoreceptors-inducing reflexive contractions of both the levator and frontalis muscles. Controversy still persists regarding the role of the mechanoreceptors in Mueller's muscle; therefore, we clinically and histologically investigated Mueller's muscle. Methods: We evaluated the role of phenylephrine administration into the upper fornix in contraction of Mueller's smooth muscle fibers and how intraoperative stretching of Mueller's muscle alters the degree of eyelid retraction in 20 patients with aponeurotic blepharoptosis. In addition, we stained Mueller's muscle in 7 cadavers with antibodies against α-smooth muscle actin, S100, tyrosine hydroxylase, c-kit, and connexin 43. Results: Maximal eyelid retraction occurred approximately 3.8 minutes after administration of phenylephrine and prolonged eyelid retraction for at least 20 minutes after administration. Intraoperative stretching of Mueller's muscle increased eyelid retraction due to its reflexive contraction. The tyrosine hydroxylase antibody sparsely stained postganglionic sympathetic nerve fibers, whereas the S100 and c-kit antibodies densely stained the interstitial cells of Cajal (ICCs) among Mueller's smooth muscle fibers. A connexin 43 antibody failed to stain Mueller's muscle. Conclusions: A contractile network of ICCs may mediate neurotransmission within Mueller's multiunit smooth muscle fibers that are sparsely innervated by postganglionic sympathetic fibers. Interstitial cells of Cajal may also serve as mechanoreceptors that reflexively contract Mueller's smooth muscle fibers, forming intimate associations with intramuscular trigeminal proprioceptive fibers to induce reflexive contraction of the levator and frontalis muscles. |
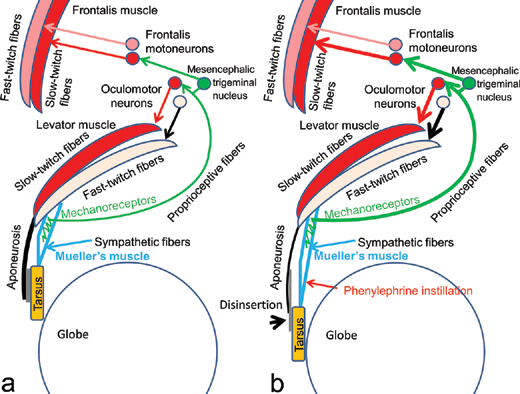
Mueller's smooth muscle fibers are serially located between the levator muscle fibers and the tarsus, under the levator aponeurosis; we have previously reported that Mueller's smooth muscle fibers are innervated by unmyelinated sympathetic efferent fibers, and furthermore, that the intramuscular connective tissues interspersed among the smooth muscle fibers are innervated by myelinated trigeminal proprioceptive afferent fibers (Fig 1a).1,2 The latter fibers function as mechanoreceptors, inducing reflexive contraction of 2 different eyelid-opening muscles, the levator and frontalis muscles. Voluntary contraction of the levator fast-twitch muscle fibers stretches the mechanoreceptors in Mueller's muscle to evoke trigeminal proprioception, thereby stimulating both the oculomotor neurons and the frontalis motoneurons to induce reflexive contraction of the levator and frontalis slow-twitch muscle fibers, respectively. This results in involuntary continuous lifting of the eyelid and eyebrow to maintain a visual field corresponding to changes in vertical gaze as a type of length servomechanism.3-9
Aponeurotic blepharoptosis is caused by disinsertion of the levator aponeurosis from the tarsus and elongated attenuation of the levator aponeurosis and underlying Mueller's muscle (Figs 1b and 2a).10-12 During eyelid opening in patients with aponeurotic blepharoptosis, the retractile force of the levator muscle is transmitted to the tarsus via the sympathetically innervated Mueller's muscle instead of the aponeurosis. It has been empirically noted that the eye will open quite normally despite total disconnection of the aponeurosis, as long as there is a normally functioning Mueller's muscle.13 Therefore, in patients with aponeurotic blepharoptosis, stretching of Mueller's muscle must induce contraction of Mueller's smooth muscle fibers for transmission of the retractile force from the levator muscle to the tarsus.
As controversy persists around the identity and physiological roles of the mechanoreceptor in Mueller's muscle, we sought to clinically and histologically investigate Mueller's muscle.
METHODS
Phenylephrine (an α1-selective agonist) was administered into the upper fornix to contract the partial Mueller's muscle in each of 20 patients (15 women and 5 men; 40.9 ± 5.2 years old) with aponeurotic blepharoptosis. Patients were made to lie in a supine position, raise their chin, and gaze downward: the upper eyelid on the side of the dominant eye was pinched for 60 seconds to detach it from the globe and create a space in the upper fornix. Two to 3 drops of 5% phenylephrine were administered into the space, and the phenylephrine was retained in this position by gravity to exclusively stimulate the unilateral posterior Mueller's smooth muscle fibers that face the conjunctiva palpebrae. Changes in the distance between the upper eyelid margin and the line between the medial and lateral canthi were measured as upper eyelid retraction distance (UERD). Measurements were taken before and subsequently 1, 2, 3, 4, 5, 10, and 20 minutes after administering phenylephrine using digital photographs on primary gaze with a 10-mm square scale (Casmatch; Dai Nippon Printing Co, Ltd, Tokyo, Japan). The average time of maximal eyelid retraction induced after phenylephrine instillation was calculated.
During aponeurotic blepharoptosis surgery, UERDs were measured upon eyelid opening after unilateral reinsertion of the levator aponeurosis to the tarsus to unilaterally desensitize the mechanoreceptors in Mueller's muscle in the 20 patients. Subsequently, the bilateral tarsi were pulled caudally as far as possible without inducing pain for 5 seconds, and within a second of eyelid opening, the UERDs were measured again. All intraoperative measurements of the UERD were made on the basis of the horizontal corneal diameter and referenced to the preoperative digital photographs with a 10-mm square scale. Before and after caudal pulling of the bilateral tarsi, changes in the UERDs of the bilateral eyelids were assessed for statistical differences between the eyelids with and without the reinsertion.
For histological investigation of Mueller's muscle, 7 specimens of unilateral Mueller's muscle were obtained from 7 Japanese cadavers (3 women and 4 men; age: 82.6 ± 6.2 years). The specimens were fixed in 10% buffered formalin and processed for routine paraffin embedding. Specimens were serially sliced along the horizontal plane (8- to 10-µm thickness). The serial sections were processed for immunohistochemical staining with the avidin-biotin-peroxidase complex method. Sections were microwaved and individual sections were incubated with primary antibodies against α-smooth muscle actin (DAKO Japan Co, Ltd, Kyoto, Japan), S100 (DAKO Japan Co, Ltd, Kyoto, Japan), tyrosine hydroxylase (PROTOS BIOTECH Co, New York), c-kit (DAKO Japan Co, Ltd., Kyoto, Japan), and connexin 43 (Spring Bioscience Co., Pleasanton, Calif); antibodies were diluted to 1:100-400 and incubated with sections at 25°C for 12 to 24 hours, followed by 2 consecutive incubations with secondary antibodies and streptavidin conjugated to horseradish peroxidase (DAKO Japan Co, Ltd). Final visualization of all sections was obtained by adding 0.01% diaminobenzidine plus 0.0015% H2O2 in 0.05 M Tris-HCL buffer, pH 7.6. In addition, toluidine blue staining was used to differentiate ICCs from mast cells with metachromasia.
Five cross-sectional areas of Mueller's muscle were randomly selected from the 7 specimens. The average number of postganglionic sympathetic nerve fibers stained positive for tyrosine hydroxylase and ICCs were counted in a microscopic field 260 µm high × 340 µm wide at 400× magnification (BX50 microscope, Olympus, Tokyo, Japan).
Data were statistically analyzed using Friedman and paired t tests. A P value less than .05 was considered to indicate a significant difference. All data are represented as mean ± SD.
RESULTS
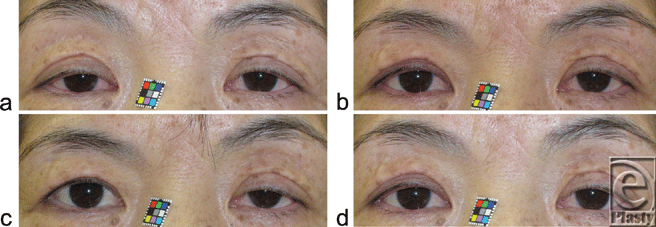
Maximal eyelid retraction due to pharmacological contraction of Mueller's smooth muscle fibers took 3.8 ± 0.8 minutes; however, initial effects were evident at 1 minute (Figs 2a-c). The induced eyelid retraction was still evident at 20 minutes after administration of phenylephrine, albeit with a gradual decline (Fig 2d). Although the eyelid retraction increased, the ipsilateral eyebrow height decreased inversely (Figs 2b-d).
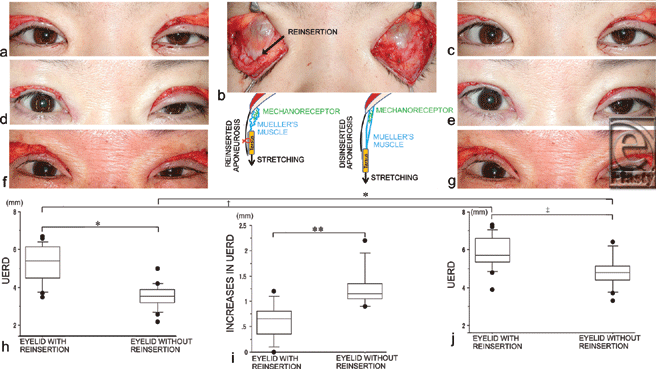
Prior to caudal pulling of the bilateral tarsi, the UERDs of the eyelids without reinsertion (3.6 ± 0.6 mm) were significantly smaller than those with reinsertion (5.3 ± 1.0 mm, P < .0001) (Figs 3a, d, f, and h). After caudal pulling of the bilateral tarsi for 5 seconds, the UERDs of the eyelids without reinsertion (4.8 ± 0.8 mm) remained significantly smaller than those with reinsertion (5.9 ± 0.9 mm, P = .0003) (Figs 3c, e, g, and i). In addition, the UERDs of the eyelids with reinsertion increased significantly after caudal pulling of the bilateral tarsi (5.9 ± 0.9 mm vs 5.3 ± 1.0 mm, P = .0001) (Figs 3h and i). The UERDs of the eyelids without reinsertion also increased significantly after caudal pulling of the bilateral tarsi (4.8 ± 0.8 mm vs 3.6 ± 0.6 mm, P < .0001) (Figs 3h and i). The increases in the UERDs of the eyelids without reinsertion after caudal pulling (1.3 ± 0.4 mm) were significantly greater than those with reinsertion (0.6 ± 0.3 mm, P = .0001) (Fig 3j).
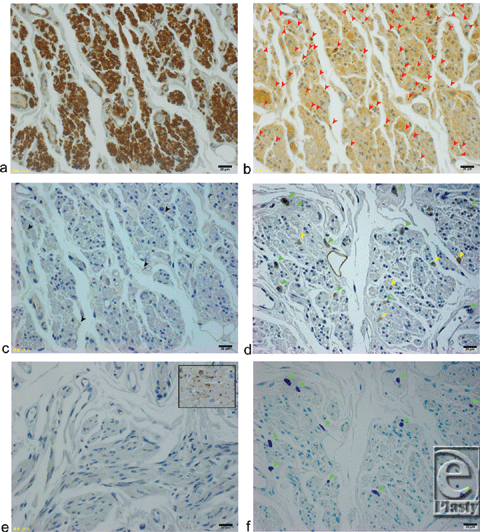
Mueller's muscle fibers were independently stained with α-smooth muscle actin antibody (Fig 4a). Schwann cells of the myelinated proprioceptive nerve fibers were densely stained with an S100 antibody (Fig 4b).1,2 A tyrosine hydroxylase antibody only sparsely stained 4.7 ± 1.1 unmyelinated postganglionic sympathetic nerve fibers (Fig 4c); the c-kit antibody, however, stained 7.3 ± 1.5 interstitial cells of Cajal (ICCs; Fig 4d) and 6.3 ± 1.2 mast cells (Fig 4f) in a microscopic field 260 µm high × 340 µm wide at 400× magnification. Mast cells were identified by staining with toluidine blue. The average number of the ICCs was significantly greater than the number of the sympathetic fibers (P = .018). The gap junctions in Mueller's smooth muscle fibers, however, were not clearly stained by a connexin 43 antibody (Fig 4e); as a control, the gap junctions in the human cardiac muscle were well stained with a connexin 43 antibody (Fig 4e, insert).
DISCUSSION
Pharmacological contraction of the posterior Mueller's smooth muscle fibers by administration of phenylephrine to the superior fornix gradually propagated to the anterior Mueller's smooth muscle fibers, and maximal eyelid retraction occurred at approximately 3.8 minutes; the whole Mueller's muscle fibers contracted sufficiently to retract the eyelid to a kind of syncytium. Pharmacological induction of eyelid retraction lasted more than 20 minutes and, however, gradually declined after the first 3.8 minutes. Pharmacological contraction of Mueller's smooth muscle fibers desensitized the mechanoreceptors among them and prevented stretching by contraction of the levator muscle; this increased ipsilateral eyelid retraction but decreased reflexive contraction of the ipsilateral levator and frontalis slow-twitch muscle fibers, resulting in decreased retraction of the contralateral eyelid (Hering's law of equal innervation to the levator muscles) and ipsilateral eyebrow height (Fig 2c). We have previously reported that pharmacological contraction of canine Mueller's smooth muscle fibers by retrograde administration of α1A-adrenoceptor agonist to the drained vein of Mueller's muscle lasted for 92 minutes.14 Other studies have demonstrated that phenylephrine injected into the femoral vein induces sustained contraction of Mueller's muscle in anesthetized rats.15 These findings suggest the involvement of a structure that mediates neurotransmission among Mueller's smooth muscle fibers and sustains their prolonged contraction.
Reinsertion of the levator aponeurosis to the tarsus resulted in significantly decreased stretching of the mechanoreceptors in Mueller's muscle upon caudal pulling of the tarsi (Fig 3b). The reflexive contraction of the levator muscle induced by caudal pulling of the tarsi was not maintained after cessation of the pulling force because of a stretch reflex in the skeletal muscle. Therefore, in the eyelid without reinsertion, we interpret the significant increase in the UERD after the cessation of caudal pulling of the tarsi as a result of prolonged reflexive contraction of Mueller's muscle. The mean maximum total excursion of the upper eyelid due to the action of Mueller's muscle was reported to be 3.0 mm, with 1.5 mm upward and 1.5 mm downward displacement from its tonic position.16 In our study, the mean increases in UERD of 1.3 mm due to the caudal pulling was consistent with the published report.
It is plausible that the reflexive contraction of Mueller's muscle is induced by stretching of Mueller's muscle and this assumption has been supported in several studies4,17-20; one study demonstrates that upgaze improves ptosis whereas downgaze worsens ptosis in acquired blepharoptosis such as aponeurotic blepharoptosis. In addition, upgaze increases stretching of Mueller's muscle, thereby resulting in its reflexive contraction, whereas downgaze decreases stretching of Mueller's muscle, thereby relaxing the muscle. The muscle bellies of the levator and Mueller's muscles function as a single serial muscle unit in an aponeurotic blepharoptosis (Fig 1b) 21; therefore, it is likely that the degree of reflexive contraction of the levator muscle is exaggerated by the degree of reflexive contraction of Mueller's muscle in patients with aponeurotic blepharoptosis.
Histologically, distributions of unmyelinated postganglionic sympathetic nerve fibers (as stained by the tyrosine hydroxylase antibody) were more sparse than expected. On the contrary, ICCs (stained by c-kit antibody) were densely stained among Mueller's smooth muscle fibers. Mast cells, which were also stained by c-kit antibody, were differentiated from ICCs with metachromasia by toluidine blue staining. Myelinated trigeminal nerve fibers, which correspond to the proprioceptive nerve fibers, were also densely stained by S100 antibody as we have previously reported.1,2 Although the connexin 43 antibody is known to stain the gap junctions in vascular smooth muscle fibers, myoepithelial smooth muscle fibers, and ICCs in the bladder and gastrointestinal tract,22-25 it stained neither Mueller's smooth muscle fibers nor ICCs. Therefore, other connexins such as connexin 26, 32, etc, may exist to connect the ICCs and Mueller's smooth muscle fibers.
Previous reports have established several functions mediated by ICCs, including (i) their role as pacemakers that actively propagate slow electrical waves in gastrointestinal muscles, resulting in their peristalsis; (ii) mediation of both inhibitory and excitatory motor neurotransmission; (iii) their role as nonneural stretch receptors in gastrointestinal muscles and the bladder, affecting both smooth muscle excitability and slow wave frequency; and finally (iv) their ability to form intimate associations with the intramuscular terminals of vagal afferents, potentially conferring a role in afferent signaling.23,24,26-37 Phenylephrine-induced contraction of posterior Mueller's smooth muscle fibers has been shown to gradually propagate to anterior Mueller's smooth muscle fibers. As a consequence, it is likely that a contractile network of ICCs may mediate neurotransmission between Mueller's multiunit smooth muscles fibers, which are sparsely innervated by postganglionic sympathetic fibers (Fig 5). In addition, intraoperative stretching of Mueller's muscle results in their reflexive contraction. Interstitial cells of Cajal may also serve as mechanoreceptors that cause their reflexive contraction (Fig 5). Interstitial cells of Cajal may also form intimate associations with intramuscular trigeminal proprioceptive fibers (stained densely by the S100 antibody), which function as mechanoreceptors, inducing reflexive contraction of the levator and frontalis muscles (Fig 5). Both c-kit-positive ICCs and c-kit-negative Mueller's smooth muscle fibers arise from mesenchymal stem cells.38-41 The major difference between ICCs and Mueller's smooth muscle fibers is the presence or absence of the c-kit gene, the protooncogene encoding a receptor tyrosine kinase,42 which dictates cellular functions such as the mediation of neurotransmission and contraction.
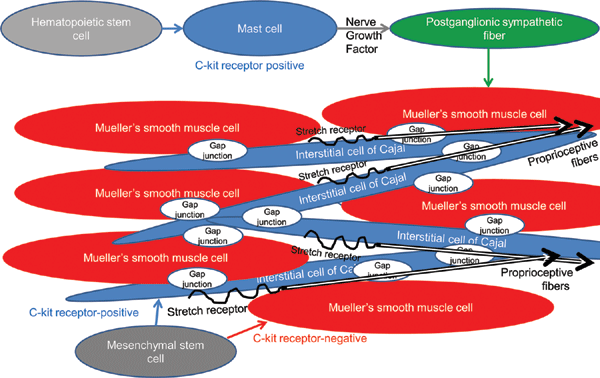 |
| Figure 5. Hypothetical relationships between Mueller's smooth muscle fibers, ICCs, post-ganglionic sympathetic efferent fibers, trigeminal proprioceptive afferent fibers, and mast cells. |
Mast cells are components of connective tissue in many regions of the body. Their functions are poorly understood; however, they are believed to release nerve growth factor43 as postganglionic sympathetic nerve fibers are unmyelinated and lack Schwan cells, which are normally required to release nerve growth factor in myelinated nerve fibers.44
CONCLUSIONS
Mechanoreceptor stretching in Mueller's muscle (which associates with both the intramuscular terminal of trigeminal proprioceptive afferents and ICCs) may induce reflexive contraction of the levator and frontalis slow-twitch muscle fibers as well as Mueller's smooth muscle fibers. Further studies are required to confirm the presence of the connexin and stretch receptors in Mueller's smooth muscle fibers.
An additional hypothesis suggests that stretching of the mechanoreceptors in Mueller's muscle increases the sympathetic tone resulting in its contraction via the trigeminal proprioceptive afferent fibers and the sympathetic efferent fibers.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science JSPA) (KAKENHI; No. 21592284).
None of the authors have any commercial associations or financial interests that may pose or create a conflict of interest with the information presented in this article.
1. Yuzuriha S, Matsuo K, Ishigaki Y, et al. Efferent and afferent innervations of Mueller's muscle related to involuntary contraction of the levator muscle: important for avoiding injury during eyelid surgery. Br J Plast Surg. 2005;58:42-52. |
2. Yuzuriha S, Matsuo K, Hirasawa C, et al. Refined distribution of myelinated trigeminal proprioceptive nerve fibres in Mueller's muscle as the mechanoreceptors to induce involuntary reflexive contraction of the levator and frontalis muscles. J Plast Reconstr Aesthet Surg. 2009;62:1403-10. |
3. Matsuo K. Stretching of the Mueller muscle results in involuntary contraction of the levator muscle. Ophthal Plast Surg. 2002;18:5-10. |
4. Hirasawa C, Matsuo K, Kikuchi N, et al. Upgaze eyelid position allows differentiation between congenital and aponeurotic blepharoptosis according to the neurophysiology of eyelid retraction. Ann Plast Surg. 2006;57:529-34. |
5. Kushima H, Yuzuriha S, Kondou S, et al. Blepharoplasty with aponeurotic fixation corrects asymmetry of the eyebrows caused by paralysis of the unilateral frontalis muscle in orientals. Scand J Plast Reconstr Surg Hand Surg. 2005;39(1):39-44. |
6. Kushima H, Matsuo K, Yuzuriha S, et al. The occipitofrontalis muscle is composed of two physiologically and anatomically different muscles separately affecting the positions of the eyebrow and hairline. Br J Plast Surg. 2005;58:681-7. |
7. Kondoh S, Matsuo K, Kikuchi N, et al. Pathogenesis and surgical correction of involuntary contraction of the occipitofrontalis muscle that causes forehead wrinkles. Ann Plast Surg. 2006;57:142-8. |
8. Matsuo K, Yuzuriha S. Frontalis suspension with fascia lata for severe congenital blepharoptosis using enhanced involuntary reflex contraction of the frontalis muscle. J Plast Reconstr Aesthet Surg. 2009;62:480-7. |
9. Ban R, Matsuo K, Osada Y, et al. Reflexive contraction of the levator palpebrae superioris muscle to involuntarily sustain the effective eyelid retraction through the transverse trigeminal proprioceptive nerve on the proximal Mueller's muscle: verification with evoked electromyography. J Plast Reconstr Aesthet Surg. 2010;63:59-64. |
10. Sultana R, Matsuo K, Yuzuriha S, et al. Disinsertion of the levator aponeurosis from the tarsus in growing children. Plast Reconstr Surg. 2000;106:563-70. |
11. Fujiwara T, Matsuo K, Kondoh S, et al. Etiology and pathogenesis of aponeurotic blepharoptosis. Ann Plast Surg. 2001;46:29-35. |
12. Matsuo K. Restration of involuntary tonic contraction of the levator muscle in patients with aponeurotic blepharoptosis or Horner syndrome by aponeurotic advancement using the orbital septum. Scand J Plast Reconstr Surg Hand Surg. 2003;37:81-9. |
13. Flowers RS. The art of eyelid and orbital aesthetics: multiracial surgical considerations. Clin Plast Surg. 1987;14:703-21. |
14. Yano S, Hirose M, Nakada T, et al. Selective α1A-adrenoceptor stimulation induces Mueller's smooth muscle contraction in an isolated canine upper eyelid preparation. Cur Eye Res. 2010;35:363-9. |
15. Bodker FS, Putterman AM, Laris A, et al. The effect of hyperthyroidism on Mueller's muscle contractility in rats. Ophthal Plast Reconstr Surg. 1997;13:161-7. |
16. Felt DP, Frueh BR. A pharmacologic study of the sympathetic eyelid tarsal muscles. Ophthal Plast Reconstr Surg. 1988;4:15-24. |
17. Patipa M. Visual field loss in primary gaze and reading gaze due to acquired blepharoptosis and visual field improvement following ptosis surgery. Arch Ophthalmol. 1992;110:63-7. |
18. Dryden RM, Kahanic DA. Worsening of blepharoptosis in downgaze. Ophthal Plast Reconstr Surg. 1992;8:126-9. |
19. Wojno TH. Downgaze ptosis. Ophthal Plast Reconstr Surg. 1993;9:83-8. |
20. Meyer DR, Rheeman CH. Downgaze eyelid position in patients with blepharoptosis. Ophthalmology. 1995;102:1517-23. |
21. Kuwabara T, Cogan DG, Johnson CC. Structure of the muscles of the upper eyelid. Arch Ophthalmol. 1975;93:1189-97. |
22. Sui GP, Rothery S, Dupont E, et al. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118-29. |
23. McCloskey KD. Interstitial cells in the urinary bladder—localization and function. Neurourol Urodyn. 2010;29:82-7. |
24. Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576(Pt 3):653-8. |
25. Seki K, Komuro T. Distribution of interstitial cells of Cajal and gap junction protein, Cx 43 in the stomach of wild-type and W/Wv mutant mice. Anat Embryol (Berl). 2002;206:57-65. |
26. Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1-130. |
27. Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369-75. |
28. Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91-7. |
29. Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-9. |
30. Torihashi S, Ward SM, Nishikawa S, et al. c-kit-Dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97-111. |
31. Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;1:112-7. |
32. Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913-8. |
33. Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578(Pt 1):33-42. |
34. Kraichely RE, Farrugia G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:245-52. |
35. Jiang Y, Bhargava V, Mittal RK. Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 2009;297:G397-405. |
36. Wang Y, Fang Q, Lu Y, Song B, Li W, Li L. Effects of mechanical stretch on interstitial cells of Cajal in guinea pig bladder. J Surg Res. 2010;164:e213-9. |
37. McCloskey KD: Interstitial cells in the urinary bladder—localization and function. Neurourol Urodyn. 2010;29:82-7. |
38. Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996;180:97-107. |
39. Torihashi S, Ward SM, Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144-55. |
40. Klüppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn. 1998;211:60-71. |
41. Hearn CJ, Young HM, Ciampoli D, Lomax AE, Newgreen D. Catenary cultures of embryonic gastrointestinal tract support organ morphogenesis, motility, neural crest cell migration, and cell differentiation. Dev Dyn. 1999;214:239-47. |
42. Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88-9. |
43. Leon A, Buriani A, Dal Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739-43. |
44. Taniuchi M, Clark HB, Johnson EM Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094-8. |
| JOURNAL INFORMATION | ARTICLE INFORMATION |
| Journal ID: ePlasty | Volume: 12 |
| ISSN: 1937-5719 | E-location ID: e13 |
| Publisher: Open Science Company, LLC | Published: February 15, 2012 |