ADVERTISEMENT
Endovascular Approach To Treat Median Arcuate Ligament Compressing Syndrome
VASCULAR DISEASE MANAGEMENT 2017;14(2):E37-E45
Abstract
Median arcuate ligament compression syndrome (MALS) should be included in the differential diagnosis of chronic abdominal pain, weight loss, and vomiting in a young patient after excluding common etiologies. Definitive radiographic and angiographic findings, along with clinical symptoms, are needed for the accurate diagnosis of MALS. Although laparoscopic resection of the ligament with or without revascularization has been the preferred management strategy, angioplasty with stenting might be undertaken in the treatment of selected patients with this condition, while ensuring adequate long-term follow-up to assess patency of the stent using either non-invasive (duplex) modalities. The presented case demonstrates the success of angioplasty and stenting and the benefit of avoiding a decompression and revascularization surgery.
The early recognition of the anatomical compression of celiac trunk by the median arcuate ligament was made in an autopsy by Lipshutz in 1917.1 The first few cases of median arcuate ligament syndrome (MALS) compressing the celiac artery were reported in 1963 by Harjola2 and in 1965 by Dunbar3 (who dubbed it the “Dunbar syndrome”). The celiac artery compression and stenosis were visualized in an angiographic imaging. Postprandial abdominal pain, nausea, vomiting, and weight loss are the common symptoms of this rare disease (Matsumoto, et al4). Some other rare symptoms include right upper-quadrant pain, occasional diarrhea,5 exercise intolerance,6 positional abdominal pain,7 bloating,8 weakness, fatigue, periumbilical pain, alternating constipation, and diarrhea,9 or sometimes even unexplained hypertension.10 MALS signs in physical examination could be a wide range, from complete benign exam to simple hiccups, generalized tenderness, abdominal bruit, and/or increased abdominal pain with forceful or prolonged expiration (due to the anatomical movement of the ligament during breathing).11
Since then, big advancements in diagnostic approaches and imaging methods have been developed and used in diagnosing MALS. On the other hand, multiple surgical approaches and endovascular approaches have been attempted, yet there is still no clear evidence12-14 about which method should be attempted first and which patient population should have the surgical option vs the endovascular option, or the hybrid approach (where they can do both surgical and endovascular at the same time).11
The Basic Pathology
The digestive system is highly vascular through mesenteric arteries and their collaterals, which makes the majority of patients asymptomatic. The three main large vessels – the celiac trunk (CT), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA) – are the most common vessels to be affected by stenosis, whether it is an atherosclerotic pathology from inside (which we see in most of the cases) and is associated with risk factors of atherosclerosis disease like age, diabetes, hypertension, dyslipidemia, and smoking, or a mechanical pathology (such as ligament compression from outside) like MALS. Usually, a stenosis of multiple vessels (>70%) leads to more prominent symptoms; however, a severe single-vessel disease can cause a mesenteric angina. Atherosclerosis disease is more common in the elderly population – usually age 60 years and older – and is predominant in the male population. However hypercoagulable state, contraception use, and other risk factors can contribute to developing mesenteric ischemia in women and younger patients. MALS is more common in women and more likely to occur in the second and fourth decades of life.6 It has been reported in patients from 13-83 years old and in both healthy, athletic patients and sedentary patients.14
The Clinical Picture
Patient symptoms are always non-specific, and the primary care or emergency doctor differential diagnosis is very wide. A strategy of focusing on the symptoms and the response to the problem are very important. Sometimes, it is very hard to find a patient with only one problem. Most often, you will have a patient with history of one or more of the following medical problems: diabetes, hypertension, gastroesophageal reflux disease, peptic ulcer disease, smoking, alcoholism, weight loss, vitamin deficiency, etc. The challenge for the physician is how to approach these new symptoms. Is the patient young or old? Is it a gastroparesis from diabetes? Is it his peptic ulcer disease? Is it alcoholic gastritis? Alcoholic chronic pancreatitis? Is it a mesenteric ischemia? Although a very thorough history will narrow down the differential diagnosis, a good number of possible etiologies will remain. A young patient with exercise intolerance is an alarming symptom. A young female patient is more confusing because irritable bowel syndrome is at the top of the differential diagnosis. Patients with past medical history of multiple hysterectomies, abdominal surgeries, small bowel obstruction, or abdominal shot wound will increase the suspicion of adhesions, which might be the cause of mesenteric artery stenosis. To date, there are no published reports of any genetic role, ethnicity preference, or familiar factor in MALS. A systematic approach is very helpful in reaching the diagnosis, considering the advancement in imaging modalities and diagnostic tests, such as computed tomography angiography (CTA), magnetic resonance arteriography (MRA), or measuring vascular speed through intravascular ultrasound (IVUS).7,13
MALS can worsen and complicate many co-morbid diseases. Healthy young male or female patients with MALS can have signs and symptoms of failure to thrive due to abdominal pain, food intolerance, and exercise intolerance, which will affect their growth and eventually their health. The older population will be at risk for malnutrition, vitamin deficiency, unnecessary polypharmacy, and bowel ischemia and bowel infection. Arterial aneurysm and degeneration of the aneurysm and a consequent rupture of this aneurysm is a known and reported complication in untreated MALS. Aneurysmal degeneration has been reported by Ducasse et al15 in pancreaticoduodenal arcades, gastroepiploic, or celiac arteries in 80% of cases of celiac compression, with a typically reported incidence of 3%-18%.
Case #1
 A 75-year-old woman with a history of well-controlled hypertension and type II diabetes mellitus, hysterectomy, and cholecystectomy presented to her primary physician with abdominal pain and unintentional weight loss of 25 lb for the last 8 months. The abdominal pain occurred intermittently, increasing with food intake and with little improvement with proton-pump inhibitors; she also complained of dyspepsia and bloating.
A 75-year-old woman with a history of well-controlled hypertension and type II diabetes mellitus, hysterectomy, and cholecystectomy presented to her primary physician with abdominal pain and unintentional weight loss of 25 lb for the last 8 months. The abdominal pain occurred intermittently, increasing with food intake and with little improvement with proton-pump inhibitors; she also complained of dyspepsia and bloating.
Her physical exam showed multiple scars in her abdomen and she was tender to deep and superficial palpation in the epigastric area that increased with long inspiration. No signs of malnutrition were apparent and the rest of her physical exam was completely benign.
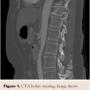
 An upper endoscopy showed erosive gastritis in the antrum and body of the stomach, and a 6 mm polyp in the cardia. Cultures were negative for H pylori. Colonoscopy showed sigmoid diverticulosis. A CT angiogram of the abdomen showed atherosclerotic disease of the abdominal aorta without aneurysmal dilatation. Hard and soft plaques were present at the celiac axis origin and there was an atypical course of the celiac axis with focal narrowing 75% at the inferior aspect of a vertically directed proximal portion and distal mild dilatation, which is most compatible with the appearance of compression by the median arcuate ligament. Superior and inferior mesenteric arteries and renal arteries demonstrated mild atherosclerotic disease, with calcifications that were negative for focal high-grade narrowing (Figure 1).
An upper endoscopy showed erosive gastritis in the antrum and body of the stomach, and a 6 mm polyp in the cardia. Cultures were negative for H pylori. Colonoscopy showed sigmoid diverticulosis. A CT angiogram of the abdomen showed atherosclerotic disease of the abdominal aorta without aneurysmal dilatation. Hard and soft plaques were present at the celiac axis origin and there was an atypical course of the celiac axis with focal narrowing 75% at the inferior aspect of a vertically directed proximal portion and distal mild dilatation, which is most compatible with the appearance of compression by the median arcuate ligament. Superior and inferior mesenteric arteries and renal arteries demonstrated mild atherosclerotic disease, with calcifications that were negative for focal high-grade narrowing (Figure 1).
Based on this study, the patient was diagnosed with compression of the celiac artery caused by MALS.
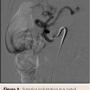
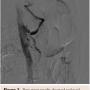 Given her age and co-morbid disease, the patient’s risk stratification was high for a moderate-to-high risk procedure; the patient was judged to be a poor surgical candidate and so the decision was made to refer her to interventional cardiology for consideration of less invasive treatment. A selective angiography of the celiac trunk was performed via the right common femoral artery with a 6 Fr system. During angiography, both inspiration and expiration films were compared; celiac artery stenosis was more prominent during expiration, and a characteristic superior indentation was noted along the proximal celiac axis (Figure 2), which led to the confirmation of MALS diagnosis. The celiac trunk was engaged with a 5 Fr, 0.035˝ Slip-cath VS 80 cm (Cook Medical), and the lesion was crossed with a 0.035˝ guidewire (Boston Scientific). Percutaneous transluminal angioplasty (PTA) of the proximal celiac trunk was performed with a 6.0 x 20 mm Mustang balloon (Boston Scientific). As expected, there was no significant angiographic improvement, so a 5.0 x 22 mm Cast stent (Atrium Medical Corporation) was deployed at 4 atm and postdilated at 10 atm. Final angiographic results showed reduced narrowing from 90% to 0%. IMA and SMA were patent (Figure 3).
Given her age and co-morbid disease, the patient’s risk stratification was high for a moderate-to-high risk procedure; the patient was judged to be a poor surgical candidate and so the decision was made to refer her to interventional cardiology for consideration of less invasive treatment. A selective angiography of the celiac trunk was performed via the right common femoral artery with a 6 Fr system. During angiography, both inspiration and expiration films were compared; celiac artery stenosis was more prominent during expiration, and a characteristic superior indentation was noted along the proximal celiac axis (Figure 2), which led to the confirmation of MALS diagnosis. The celiac trunk was engaged with a 5 Fr, 0.035˝ Slip-cath VS 80 cm (Cook Medical), and the lesion was crossed with a 0.035˝ guidewire (Boston Scientific). Percutaneous transluminal angioplasty (PTA) of the proximal celiac trunk was performed with a 6.0 x 20 mm Mustang balloon (Boston Scientific). As expected, there was no significant angiographic improvement, so a 5.0 x 22 mm Cast stent (Atrium Medical Corporation) was deployed at 4 atm and postdilated at 10 atm. Final angiographic results showed reduced narrowing from 90% to 0%. IMA and SMA were patent (Figure 3).
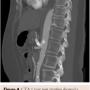 A few days after the procedure, the patient’s gastrointestinal symptoms started resolving; first, her pain was completely gone and then her appetite started to improve. At 1-year follow-up, she remained asymptomatic and regained 25 lb. Repeat CTA for right upper-quadrant pain and elevated lactic acid showed a significant distention of the common bile duct and mild intrahepatic biliary duct dilatation. The CT angiogram also showed a possible kinked stent in the celiac trunk; however, the enhancement of the distal celiac branches were consistent with patency and the patient stayed asymptomatic after the biliary stasis episode resolved (Figure 4). At 2-year follow-up exam, she had maintained her normal weight and remained completely asymptomatic.
A few days after the procedure, the patient’s gastrointestinal symptoms started resolving; first, her pain was completely gone and then her appetite started to improve. At 1-year follow-up, she remained asymptomatic and regained 25 lb. Repeat CTA for right upper-quadrant pain and elevated lactic acid showed a significant distention of the common bile duct and mild intrahepatic biliary duct dilatation. The CT angiogram also showed a possible kinked stent in the celiac trunk; however, the enhancement of the distal celiac branches were consistent with patency and the patient stayed asymptomatic after the biliary stasis episode resolved (Figure 4). At 2-year follow-up exam, she had maintained her normal weight and remained completely asymptomatic.
Case #2
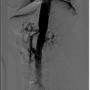 A 55-year-old Caucasian women with medical history of diverticulosis, cervical spondylosis, cervical spinal stenoses at level C3 and C4, prior caesarean section, and past smoker presented with abdominal pain for the last 2 months. The patient described her abdominal pain as sharp and colicky, with varying locations including right upper quadrant (RUQ), peri-umbilical, and suprapubic. Pain was exacerbated with meals and she had a 15 to 20 lb weight loss due to poor oral intake. She also had nausea, vomiting, bloating, gas, and chronic constipation. She denied fevers, diarrhea, chest pain, shortness of breath, hematuria, and hematochezia. The patient stated that the pain initially started as suprapubic pressure 3 months prior, for which she was treated for cystitis with a 1-week course of nitrofurantoin and a 3-week course of ciprofloxacin. Urology evaluated the patient due to persistent symptoms and both bladder scan and cystoscopy were unremarkable. She presented to the emergency department 2 months later for persistent abdominal pain, with the pain worst at this time in the RUQ. Her physical exam showed soft abdomen with positive bowel sounds, generalized tenderness more in the RUQ but no guarding, rigidity, or distention. She had CT of the abdomen and pelvis, which was negative for acute intraabdominal pathologies; the patient was discharged home with a follow-up appointment to see a gastroenterologist. The patient visited the gastroenterologist, who offered her a screening colonoscopy that showed evidence of diverticulosis with stricture but no diverticulitis, no polyps, or masses. The patient came in again for follow-up visit for persistent nausea, vomiting, and abdominal pain. This time, a CT angiogram of the abdomen and pelvis revealed a high-grade short-segment stenosis at the ostium of her celiac trunk. Given the relatively smooth margins of the celiac trunk, the stenosis most likely was due to MALS.
A 55-year-old Caucasian women with medical history of diverticulosis, cervical spondylosis, cervical spinal stenoses at level C3 and C4, prior caesarean section, and past smoker presented with abdominal pain for the last 2 months. The patient described her abdominal pain as sharp and colicky, with varying locations including right upper quadrant (RUQ), peri-umbilical, and suprapubic. Pain was exacerbated with meals and she had a 15 to 20 lb weight loss due to poor oral intake. She also had nausea, vomiting, bloating, gas, and chronic constipation. She denied fevers, diarrhea, chest pain, shortness of breath, hematuria, and hematochezia. The patient stated that the pain initially started as suprapubic pressure 3 months prior, for which she was treated for cystitis with a 1-week course of nitrofurantoin and a 3-week course of ciprofloxacin. Urology evaluated the patient due to persistent symptoms and both bladder scan and cystoscopy were unremarkable. She presented to the emergency department 2 months later for persistent abdominal pain, with the pain worst at this time in the RUQ. Her physical exam showed soft abdomen with positive bowel sounds, generalized tenderness more in the RUQ but no guarding, rigidity, or distention. She had CT of the abdomen and pelvis, which was negative for acute intraabdominal pathologies; the patient was discharged home with a follow-up appointment to see a gastroenterologist. The patient visited the gastroenterologist, who offered her a screening colonoscopy that showed evidence of diverticulosis with stricture but no diverticulitis, no polyps, or masses. The patient came in again for follow-up visit for persistent nausea, vomiting, and abdominal pain. This time, a CT angiogram of the abdomen and pelvis revealed a high-grade short-segment stenosis at the ostium of her celiac trunk. Given the relatively smooth margins of the celiac trunk, the stenosis most likely was due to MALS.
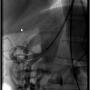 A discussion with the patient regarding treatment options was done, and considering her comorbidities and patient preference, the decision was made for minimally invasive endovascular stenting. A selective angiography of the celiac trunk was performed via the left brachial artery access using a 5 Fr, 125 cm, MP A-2 catheter (Merit Medical Systems, Inc) over the Supracore guidewire (Abbot Vascular). A 90% stenosis in the celiac trunk was identified, and SMA and IMA had no significant stenoses (Figure 5).
A discussion with the patient regarding treatment options was done, and considering her comorbidities and patient preference, the decision was made for minimally invasive endovascular stenting. A selective angiography of the celiac trunk was performed via the left brachial artery access using a 5 Fr, 125 cm, MP A-2 catheter (Merit Medical Systems, Inc) over the Supracore guidewire (Abbot Vascular). A 90% stenosis in the celiac trunk was identified, and SMA and IMA had no significant stenoses (Figure 5).
PTA of the proximal celiac trunk was performed with a 6.0 x 20 mm Mustang balloon. As expected, there was no significant angiographic improvement post balloon dilatation (Figure 6). A 6.0 x 16 mm Atrium stent was deployed across the target lesion at 12 atm. There was 0% stenosis post stent placement (Figure 7).
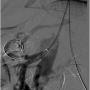 Immediately after the procedure, the patient’s abdominal pain resolved, and her appetite returned the following day. After 1 month, she started having some epigastric abdominal pain related to food. A repeat CT angiogram showed complete patency of her stent (Figure 8). An abdominal ultrasound showed a cholelithiasis and gall bladder polyp. At 6-month follow-up, the patient had regained 13 lb and had no problems with eating her regular meals. The patient was followed for the next 4 years, and remained asymptomatic for the entire time.
Immediately after the procedure, the patient’s abdominal pain resolved, and her appetite returned the following day. After 1 month, she started having some epigastric abdominal pain related to food. A repeat CT angiogram showed complete patency of her stent (Figure 8). An abdominal ultrasound showed a cholelithiasis and gall bladder polyp. At 6-month follow-up, the patient had regained 13 lb and had no problems with eating her regular meals. The patient was followed for the next 4 years, and remained asymptomatic for the entire time.
Diagnostics: The Present and the Future
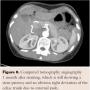 MALS is a diagnosis by exclusion usually due to rarity of the disease. Normally, the gastroenterology work-up take place first to rule out gastroesophageal reflux disease, peptic ulcer disease, gastric cancer, or other pathology mimicking the symptoms of MALS. Mesenteric ischemia is next in the work-up for elderly patients with risk factors, and irritable bowel syndrome, psychosocial, and stress-related symptoms should be evaluated as well (especially in young patients). There are many different diagnostic possibilities. With persistent symptoms, the first reasonable investigation could be functional color duplex sonography with breathing maneuvers, since it is less invasive in measuring the stenosis by measuring the vascular velocity (the greater the stenosis, the higher the velocity). For better visualization, CTA and magnetic resonance angiography are better options; however, no superiority between CTA and duplex ultrasound has been reported.16 Other, less frequently used diagnostic investigations, such as provocation of a steal phenomenon by direct vasodilator injection into the celiac trunk or SMA, intra-arterial pressure measurement, intravascular ultrasound, and gastric exercise tonometry may add to the diagnosis of MALS.17
MALS is a diagnosis by exclusion usually due to rarity of the disease. Normally, the gastroenterology work-up take place first to rule out gastroesophageal reflux disease, peptic ulcer disease, gastric cancer, or other pathology mimicking the symptoms of MALS. Mesenteric ischemia is next in the work-up for elderly patients with risk factors, and irritable bowel syndrome, psychosocial, and stress-related symptoms should be evaluated as well (especially in young patients). There are many different diagnostic possibilities. With persistent symptoms, the first reasonable investigation could be functional color duplex sonography with breathing maneuvers, since it is less invasive in measuring the stenosis by measuring the vascular velocity (the greater the stenosis, the higher the velocity). For better visualization, CTA and magnetic resonance angiography are better options; however, no superiority between CTA and duplex ultrasound has been reported.16 Other, less frequently used diagnostic investigations, such as provocation of a steal phenomenon by direct vasodilator injection into the celiac trunk or SMA, intra-arterial pressure measurement, intravascular ultrasound, and gastric exercise tonometry may add to the diagnosis of MALS.17
Treatment History: Modality, Method, and Outcomes
Since 1963, there have been tremendous advancements in medicine and surgical tools and approaches. Surgery was the treatment of choice until the endovascular era dawned. Sophisticated imaging helped identify and establish diagnoses accurately and added to patient management based upon the individual patient’s health and risk factors. The open surgical option was the first definitive treatment, and was then proceeded by laparoscopic surgery, which was more appealing to surgeons, patients, and the health-care community at large, with a trend toward less-invasive procedures. The high rate of postoperative complications with the laparoscopic option encouraged interventional radiologists and interventional cardiologists to attempt revascularization of the restenosed vessels with complete symptom withdrawal, which they termed the “hybrid” treatment. Solo endovascular treatment was successfully reported in a few cases; however, failures were also reported, with crushed and kinked stents. The advancements in endovascular catheters, angioplasty, and stent technology are promising and may eventually completely replace the surgical option.18 The reason for the failure is not in-stent restenosis; rather, it is the external mechanical power of the adhesions post operation in the surgical/hybrid model or the arcuate ligament itself in the solo endovascular model. As we advance with endovascular technology and create stronger, more stable stents, the solo endovascular model will be more practical (especially for the high surgical risk population), and will provide better quality of life and fewer complications for patients, and less financial burden on the health-care system.
Conclusion
MALS should be included in the differential diagnosis of chronic abdominal pain, weight loss, and vomiting in a young patient after excluding common etiologies. Definitive radiographic and angiographic findings, along with clinical symptoms, are needed for the accurate diagnosis of MALS. Although laparoscopic resection of the ligament with or without revascularization has been the preferred management strategy, angioplasty with stenting might be undertaken in the treatment of selected patients with this condition, while ensuring adequate long-term follow-up to assess stent patency using non-invasive (duplex) modalities. The two cases presented herein demonstrate the success of angioplasty and stenting and the benefit of avoiding a decompression and revascularization surgery, reflected in the fact that the patients had immediate clinical resolution of symptoms and continued to be symptom free at long-term follow-up, with documented stent patency.
From Mercy Hospital Medical Center, Internal Medicine Department and Wayne State University School of Medicine, Department of Family Medicine and Public Health.
Disclosure: The authors have completed and returned the ICMJE form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Address for correspondence: Dhafer Salem, MD, MPH, 401 East 32nd Street, Chicago IL 60616. Email: salemdhafer@yahoo.com
REFERENCES
- Lipshutz B. A composite study of the coelac axis artery. Ann Surg. 1917;65:159-169.
- Harjola PT. (1963). A rare obstruction of the celiac artery. Report of a case. Ann Chir Gynaecol Fenn. 1963;52:547-550.
- Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965;95:731-744.
- Matsumoto AH, Angle JF, Spinosa DJ, et al. Percutaneous transluminal angioplasty and stenting in the treatment of chronic mesenteric ischemia: results and long-term followup. J Am Coll Surg. 2002;194(1 Suppl):S22-S31.
- Lee JJ, Mills JL. Chronic mesenteric ischemia from diaphragmatic compression of the celiac and superior mesenteric arteries. Ann Vasc Surg. 2016;30:311.e5-e8. Epub 2015 Nov 2.
- Haskins IN, Harr JN, Brody F. Exercise-related transient abdominal pain secondary to median arcuate ligament syndrome: a case report. J Sports Sci. 2015;1-4.
- de Lara FV, Higgins C, Hernandez-Vila EA. Median arcuate ligament syndrome confirmed with the use of intravascular ultrasound. Tex Heart Inst J. 2014;41:57-60.
- Delis KT, Gloviczki P, Altuwaijri M, McKusick MA. Median arcuate ligament syndrome: open celiac artery reconstruction and ligament division after endovascular failure. J Vasc Surg. 2007;46:799-802.
- Gunduz Y, Asil K, Aksoy YE, Tatli Ayhan L. Clinical and radiologic review of uncommon cause of profound iron deficiency anemia: median arcuate ligament syndrome. Korean J Radiol. 2014;15:439-442.
- Arazinska A, Polguj M, Wojciechowski A, Trebinski L, Stefanczyk L. An unusual case of left renal artery compression: a rare type of median arcuate ligament syndrome. Surg Radiol Anat. 2016;38:379-382. Epub 2015 May 5.
- Palmer OP, Tedesco M, Casey K, Lee JT, Poultsides GA. Hybrid treatment of celiac artery compression (median arcuate ligament) syndrome. Dig Dis Sci. 2012;57:1782-1785.
- Columbo JA, Trus T, Nolan B, et al. Contemporary management of median arcuate ligament syndrome provides early symptom improvement. J Vasc Surg. 2015;62:151-156.
- Tracci MC. Median arcuate ligament compression of the mesenteric vasculature. Tech Vasc Interv Radiol. 2015;18:43-50.
- Gibbons CP, Roberts DE. Endovascular treatment of chronic arterial mesenteric ischemia: a changing perspective? Semin Vasc Surg. 2010;23:47-53.
- Ducasse E, Roy F, Chevalier J, et al. Aneurysm of the pancreaticoduodenal arteries with a celiac trunk lesion: current management. J Vasc Surg. 2004;39:906-911.
- Bagley J, Stamile E, Berry JL, DiGiacinto D. Case report: median arcuate ligament syndrome diagnosed with computed tomography and Doppler ultrasonography. Radiol Technol. 2015;86:238-245.
- Czihal M, Banafsche R, Hoffmann U, Koeppel T. Vascular compression syndromes. Vasa. 2015;44:419-434.
- Doshi P. Celiac artery stenting. Endovascular Today. March, 2009.











