Zero-Contrast Revascularization of a Chronic Total Occlusion in the Superficial Femoral Artery Using Intravascular OCT-Guided Crossing and Directional Atherectomy in a Patient With Chronic Kidney Disease
The treatment and management of patients with peripheral artery disease (PAD) and chronic kidney disease (CKD) remains an important clinical challenge. According to the Centers for Disease Control and Prevention, more than 20 million people in the United States currently suffer from varying levels of CKD.1 As it relates to peripheral vascular disease, patients with impaired renal function have a greater than two-fold risk of developing PAD, with one cross-sectional analysis stating that 24% of all CKD patients suffer from PAD.2 Furthermore, data from the National Health and Nutrition Examination Survey (NHANES) revealed that not only is risk of PAD continuing to grow in the United States, but approximately 5% of all adults 40 years and older suffer from this disease.3 Although it is clear that incidence rates of PAD in CKD patients will continue to rise, surgical treatment methods for patients undergoing lower-extremity revascularization have yet to be established due to the reliance on iodinated intravascular contrast for vessel and chamber imaging. With contrast-induced acute kidney injury (CI-AKI) representing the most common cause of AKI following intravenous contrast media administration,4 a preventive approach may be the best way to strategize revascularization of patients at high risk for CI-AKI.
In an attempt to improve both acute and long-term outcomes for endovascular therapies, interventionists are increasingly adopting intravascular imaging, which has demonstrated clinical benefits during percutaneous coronary intervention for the diagnoses and characterization of plaque burden, location, and to gauge optimal therapeutic endpoints.5,6 Recently, optical coherence tomography (OCT)-guided revascularization technologies have been approved for treatment of PAD, including the Ocelot OCT-guided chronic total occlusion (CTO) crossing catheter (Avinger) and the Pantheris OCT-guided atherectomy catheter (Avinger). The combination of intravascular, light-based imaging with real-time therapeutic capability enables reduced fluoroscopy and contrast-free revascularization of high-risk patient populations.
Herein, we report a CKD patient presenting with elevated creatinine (3.8 mg/dL) and a 280 mm severely calcified CTO of the superficial femoral artery (SFA), which was safely and effectively revascularized without contrast using OCT-guided CTO crossing and OCT-guided directional atherectomy.
Case Description
A 65-year-old female presenting with lower right-extremity rest pain was referred with category 3 Rutherford intermittent claudication with lifestyle-limiting symptoms. Past medical history was significant for coronary artery disease (CAD), PAD, and CKD. Diagnostic duplex ultrasound indicated a calcified CTO in the right SFA. Due to progressive symptoms, the patient was brought in for percutaneous diagnostic and therapeutic intervention using carbon-dioxide (CO2) angiography.

 Access was obtained in the left common femoral artery using a micropuncture access set (Cook Medical), followed by placement of a 6 Fr sheath. A 7 Fr Pinnacle Destination sheath (Terumo Corporation) was then advanced over a stiff-angled Glidewire (Terumo Corporation) across the aortoiliac bifurcation and into the right SFA. Diagnostic angiography using CO2 confirmed ultrasound findings of a long (280 mm), calcified, right SFA-CTO with reconstitution of the distal SFA via collaterals from the deep femoral artery and 3-vessel run-off into the right foot (Figure 1). A Prowater wire (Abbott Vascular) was advanced into the proximal cap of the right SFA-CTO. The OCT-guided Ocelot crossing catheter (Figure 2) was advanced over the wire and used to cross the CTO up to the distal cap, where severe calcification and a fibrotic cap prevented direct entry into the distal true lumen on the first attempt. Access was then obtained in the right posterior tibial artery using ultrasound guidance with placement of a 6 Fr sheath in the right posterior tibial artery. The Ocelot catheter was advanced retrograde, successfully crossing the distal cap via the true lumen into the communicating antegrade channel created previously and the retrograde wire was advanced and externalized into the antegrade sheath.
Access was obtained in the left common femoral artery using a micropuncture access set (Cook Medical), followed by placement of a 6 Fr sheath. A 7 Fr Pinnacle Destination sheath (Terumo Corporation) was then advanced over a stiff-angled Glidewire (Terumo Corporation) across the aortoiliac bifurcation and into the right SFA. Diagnostic angiography using CO2 confirmed ultrasound findings of a long (280 mm), calcified, right SFA-CTO with reconstitution of the distal SFA via collaterals from the deep femoral artery and 3-vessel run-off into the right foot (Figure 1). A Prowater wire (Abbott Vascular) was advanced into the proximal cap of the right SFA-CTO. The OCT-guided Ocelot crossing catheter (Figure 2) was advanced over the wire and used to cross the CTO up to the distal cap, where severe calcification and a fibrotic cap prevented direct entry into the distal true lumen on the first attempt. Access was then obtained in the right posterior tibial artery using ultrasound guidance with placement of a 6 Fr sheath in the right posterior tibial artery. The Ocelot catheter was advanced retrograde, successfully crossing the distal cap via the true lumen into the communicating antegrade channel created previously and the retrograde wire was advanced and externalized into the antegrade sheath.
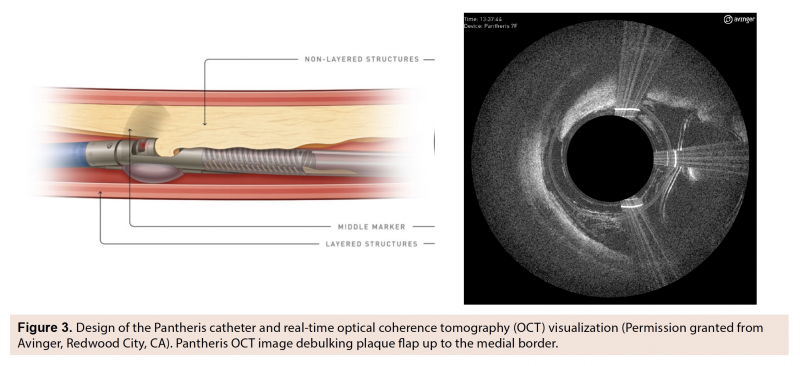
Using OCT guidance, directional atherectomy of the left SFA was successfully performed with a Pantheris catheter (Figure 3), which was advanced into the proximal common femoral artery using live OCT-guided imaging following placement of a 6.0 mm distal embolic protection device in the distal popliteal artery. Three passes were made with the catheter across the SFA-CTO, retrieving a large volume of atheroma. OCT highlighted several zones of preserved arterial lumen within the CTO. Plaque burden was mostly eccentric, spiraling throughout the artery, with the exception of dense concentric fibrous disease at the distal cap. Following debulking, a 5.0 x 120 mm In.Pact balloon (Medtronic) was delivered to the lesion site and inflated. Final CO2 angiography confirmed excellent angiographic results, with no dissections and brisk flow throughout the SFA, as well as preserved 3-vessel run-off into the right lower extremity (Figure 4).
 Zero contrast was used for the revascularization procedure. Total radiation for the procedure was measured at 0.3 Gy. The body mass index (BMI)-adjusted dose-area product for radiation dosage was 56.5 Gy•cm2. The patient was discharged home the same day without complications.
Zero contrast was used for the revascularization procedure. Total radiation for the procedure was measured at 0.3 Gy. The body mass index (BMI)-adjusted dose-area product for radiation dosage was 56.5 Gy•cm2. The patient was discharged home the same day without complications.
Discussion
CKD is a major medical issue that requires expert care and patient management in the interventional setting. To date, AKI associated with administration of iodinated contrast media is the third most common cause of renal failure in hospitalized patients.7 The number of patients at risk for contrast-induced nephropathy (CIN) has been increasing due to the increased prevalence of CKD, increasing number of diabetics, and rising elderly populations.8 Currently, CKD patients represent 20% of all PAD patients requiring intervention, a number expected to grow alongside diabetes and aging.9
Specifically pertaining to lower-extremity revascularization, complications of CIN include renal failure that may require dialysis, and it is suspected of increasing short-term and long-term morbidity and mortality rates. Current endovascular solutions for treating PAD lesions rely heavily on contrast and x-ray angiography for visualization when diagnosing lesion characteristics and directing subsequent therapy. Accordingly, CKD patients are often medically managed until they develop pervasive critical limb ischemia (CLI) or are routed to primary open surgical bypass and amputation.
OCT-guided therapies enable a solution for lower-extremity revascularizations using no or limited contrast, greatly reducing the risk for CIN and co-morbid exacerbations in patients with CKD. Avoiding the use of contrast agents is considered to be the best preventive method for patients with severe CKD, and the use of OCT offers an effective solution that can be applied to CKD patients and those with allergies to iodinated contrast media. Lastly, the safety profile of OCT guidance shifts site-of-service to the outpatient and office-based laboratory settings, minimizing inpatient costs and resources typically consumed by this patient population.
This case report demonstrates a novel, contrast-free revascularization of a severely calcified SFA-CTO in a patient suffering from CKD. This is the first report of OCT-guided therapies being used in combination with CO2 angiography for patients suffering from renal insufficiency. In this case, the visualization provided by the Ocelot and Pantheris OCT-guided catheters assisted the operators to safely and effectively revascularize a complex SFA-CTO lesion in a patient with CKD.
Conclusion
OCT-guided CTO crossing and directional atherectomy, combined with CO2 angiography, provides a safe, effective, and contrast-free therapeutic solution for patients suffering from PAD and CKD. n
Editor’s Note
Disclosure: The author has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr George discloses consulting fees from Avinger outside the submitted work.
Address for correspondence: Jon C. George, MD, Einstein Medical Center, 5501 Old York Road, 3rd Floor Levy, Philadelphia, PA 19141. Email: jcgeorgemd@hotmail.com
References
1. National Chronic Kidney Disease Fact Sheet, 2014. In: Control CfD, ed2014.
2. O’Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2004;109:320-323.
3. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738-743.
4. Sadat U, Usman A, Boyle JR, Hayes PD, Solomon RJ. Contrast medium-induced acute kidney injury. Cardiorenal Med. 2015;5:219-228.
5. Tanaka A, Imanishi T, Kitabata H, et al. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur Heart J. 2009;30:1348-1355.
6. Terashima M, Kaneda H, Suzuki T. The role of optical coherence tomography in coronary intervention. Korean J Intern Med. 2012;27:1-12.
7. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419-1428.
8. Merschen RJ. An overview of chronic kidney disease and useful strategies for clinical management. Cath Lab Digest. 2012;20(3).
9. Garimella PS, Hirsch AT. Peripheral artery disease and chronic kidney disease: clinical synergy to improve outcomes. Adv Chronic Kidney Dis. 2014;21:460-471.












