Update on the Gore SCAFFOLD Clinical Study Evaluating the Gore Mesh-Covered Carotid Stent
Despite the use of proximal and distal cerebral protection devices, carotid artery stenting has the risk of distal embolization related to plaque protrusion through the stent struts.
Second-generation mesh-covered carotid stents offer the potential advantage of improved plaque stabilization during carotid stenting procedure. This feature should reduce the risk of procedural and post-procedural neurological events due to plaque embolization. The Gore Carotid Stent is designed as a flexible open cell nitinol stent, with a 500 um pore ePTFE lattice on the outside to stabilize plaque on the wall of the artery. The device is coated with the CBAS heparin bioactive surface to reduce the risk of thrombosis (Figure 1). 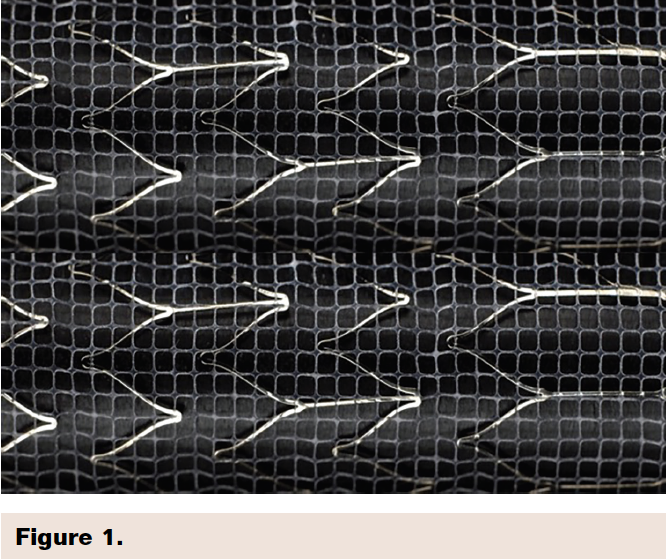
The SCAFFOLD study was initiated in 2013 for treatment of 312 high risk asymptomatic and symptomatic patients with carotid artery stenosis in up to 50 US sites. The trial had a 2-stage design, with the initial 100 patients requiring FDA review of 6-month follow-up data prior to proceeding with the second stage. A patient selection committee was formed to enforce trial inclusion/exclusion criteria in the second stage. The trial successfully completed enrollment on Dec. 2, 2016, and 30-day MAE data have been submitted for publication.
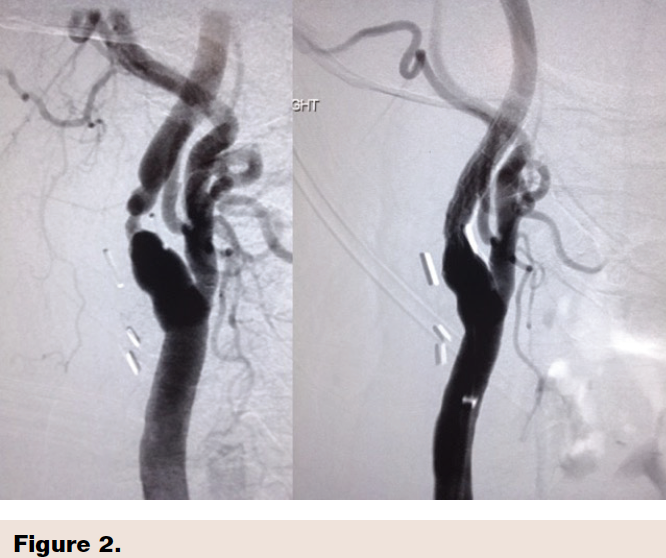
Case example with before and after CAS using the Gore Carotid stent showing excellent clinical result.(Figure 2)