Thoracic Aortic Endografting: Status Report 2012
The transformation of the field of thoracic aortic surgery began with the performance of the first stent graft procedure for repair of a descending thoracic aortic aneurysm (TAA) by Dake et al in 1992.1 It took place in the wake of the history-making first endovascular aneurysm repair (EVAR) procedure by Parodi et al in 1990, and Volodos’ first-ever stent graft aortic repair in 1986.2
The incipient thoracic endovascular aortic repair (TEVAR) field was slow to develop at first, lagging behind developments on the EVAR side for several years. Nonetheless, the emerging techniques for the thoracic aorta were received with wide enthusiasm and almost immediate embrace by specialists and the medical community at large because of the realities of surgical treatment that is viewed as maximally invasive, and often accompanied by a high rate of complication and even death. The perception of a poorly served patient population thus evolved over the years, providing great impetus for the creation of less invasive therapies.
Thoracic Aortic Aneurysm
TAA is a serious disease, and often fatal as patients face a rather limited 20% to 54% 5-year survival expectation (due to aneurysm rupture).3,4 The incidence is reported in 10.4 per 100,000 people/year,5 or approximately 30,000 new cases each year in the U.S. alone. The corresponding number for abdominal aortic aneurysm (AAA) is 200,000, reflecting its significantly higher incidence. Like AAA, TAA is asymptomatic in 95% of cases or more.
Elective repair should be considered for all 5.5 cm TAA as they carry an annual rupture risk of 15%.4,6,7 The rupture rate is reported to be 3.5 per 100,000 people/year, considerably lower than the rate of ruptured AAA. It is interesting and intriguing that the incidence of acute aortic dissection and ruptured TAA are almost identical.8 Overall, the total mortality for ruptured thoracic aneurysms approaches 97% among those reaching the hospital alive.9
Anatomic location and extent designate TAAs: ascending, arch, descending, and thoracoabdominal aortic aneurysms. Aneurysms of the descending thoracic aorta are most common (30%-40%). Aortic aneurysms have the same pathogenesis and essentially, the same risk factors: male gender, advanced age, cigarette smoking, atherosclerosis, hypertension, and genetic predisposition. Smoking is by far the most significant modifiable risk factor.10 Historically, the label atherosclerotic was applied to most thoracic and abdominal aneurysms, but today we know that the more appropriate term is degenerative. Loss of collagen and elastin in the wall of the aorta constitute the pathogenetic signatures of aneurysm formation. It is of interest that for unclear reasons there is an apparent cross-link between TAA disease and intracranial aneurysms, to the extent that some experts suggest screening and cerebrovascular imaging on thoracic aneurysm patients.
The Yale database (3,000+ patients) has produced invaluable information on the epidemiology and nature of TAA disease,11 chiefly the following:
- Thoracic aneurysmal disease tends to be genetic in nature with a predominantly autosomal dominant inheritance;
- Matrix metalloproteinase enzymes are quite active;
- Aortic wall tension and diameter go hand-in-hand, approaching the tensile limits of aortic tissue at a 6.0 cm diameter;
- Extreme physical exertion (ie, heavy lifting) and emoting can precipitate occurrence of acute aortic dissection.
An increase in the true incidence of TAA has been suggested by recent evidence.8,12,13 TAA growth can be described – generally – as slow and indolent at approximately 0.3 cm per year in the descending thoracic aorta and 0.1 cm per year in the ascending. Very rapid enlargement is often associated with an intercurrent dissection.14 Hinge points of aortic diameter progression (at which rupture or dissection are likely to occur) have emerged:11 6.0 cm in the ascending and 7.0 cm in the descending. They seem to represent the sizes where the wall tension approaches or exceeds the elastic limits of the aortic wall.14 Therefore, it is possible to prevent death from rupture of the thoracic aorta by undertaking repair before it reaches a dangerous size: the 5.5 cm diameter threshold emerges as most reasonable before intervention and repair can be performed in the majority of such patients.
More aggressive treatment guidelines can be justified in patients at greater risk such as those with Marfan syndrome, bicuspid aortic valve, and with a family history of aortic dissection. Naturally, patients with truly symptomatic aneurysms (5% or less of the TAA population) should be offered repair regardless of size.
 Surgical treatment of TAA was reported as early as 1951.15 Improvements in surgical techniques and perioperative care over the past 3 decades have allowed skilled specialized aortic surgeons to perform complex thoracic aortic surgery with excellent results and safety. Remarkably, such capabilities are available in only a few centers around the world.16 Additionally, a considerable number of patients are unlikely surgical candidates because of medical reasons, all of which explain the rapid rise of TEVAR and its transformational influence.
Surgical treatment of TAA was reported as early as 1951.15 Improvements in surgical techniques and perioperative care over the past 3 decades have allowed skilled specialized aortic surgeons to perform complex thoracic aortic surgery with excellent results and safety. Remarkably, such capabilities are available in only a few centers around the world.16 Additionally, a considerable number of patients are unlikely surgical candidates because of medical reasons, all of which explain the rapid rise of TEVAR and its transformational influence.
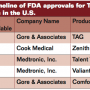
 Four FDA-approved thoracic stent graft devices are commercially available in the U.S. currently: Gore TAG, Cook TX2, Medtronic Talent, and Medtronic Valiant (Table 1). A fifth device, the Bolton Relay stent graft, completed pivotal trial enrollment and awaits regulatory approval, which is anticipated this year. They differ in several important ways (Table 2). The on-label approved indication for each is the treatment of fusiform and saccular aneurysms of the descending thoracic aorta and penetrating aortic ulcers (PAU). Regulatory approval was based on the 1-year results achieved in the various trials designed and conducted for such purpose.17-20
Four FDA-approved thoracic stent graft devices are commercially available in the U.S. currently: Gore TAG, Cook TX2, Medtronic Talent, and Medtronic Valiant (Table 1). A fifth device, the Bolton Relay stent graft, completed pivotal trial enrollment and awaits regulatory approval, which is anticipated this year. They differ in several important ways (Table 2). The on-label approved indication for each is the treatment of fusiform and saccular aneurysms of the descending thoracic aorta and penetrating aortic ulcers (PAU). Regulatory approval was based on the 1-year results achieved in the various trials designed and conducted for such purpose.17-20
TEVAR developments transformed the thoracic aortic surgery landscape. In 2010, Walker et al published a retrospective review of TAA repair rates in the U.S. from 2000 to 2007:21 The open-repair rate increased from 3.3 per million in 2000-2002 up to 5.6 per million in 2003 (when multislice CT scanners were introduced). The TEVAR repair rate changed considerably from 1.2 per million in 2005 to 6.1 repairs per million in 2006, following FDA approval of the first thoracic stent-graft (Gore TAG).
Thoracic technologies progressed slowly during the initial “infancy phase” in the 1990s and early 2000s, but are now advancing more rapidly and accumulating important clinical evidence. Limitations remain, though, which include:
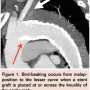 Poor conformity to the knuckle of the distal arch with resultant bird-beaking (Figure 1) has been recognized as an important problem for some time. It can lead to malapposition to the aortic wall, failure of proximal seal, a proximal type I endoleak, poor fixation, and even graft collapse that can be potentially catastrophic. Recent device designs and iterations as introduced with the Valiant Captivia (Medtronic), Zenith TX2 Pro-Form (Cook), and Conformable TAG (Gore) have improved on earlier stent graft performance and may have largely overcome such shortcomings.
Poor conformity to the knuckle of the distal arch with resultant bird-beaking (Figure 1) has been recognized as an important problem for some time. It can lead to malapposition to the aortic wall, failure of proximal seal, a proximal type I endoleak, poor fixation, and even graft collapse that can be potentially catastrophic. Recent device designs and iterations as introduced with the Valiant Captivia (Medtronic), Zenith TX2 Pro-Form (Cook), and Conformable TAG (Gore) have improved on earlier stent graft performance and may have largely overcome such shortcomings.- Profile remains a big issue as currently available devices still feature very large delivery systems. New developments with <22 Fr systems are eagerly awaited.
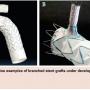 Branched technologies represent one of the next important phases in TEVAR, and a notorious target of ongoing research and development efforts everywhere. In future years, multiple iterations and designs will likely become available (Figure 2). In the interim, clinical application of various adjunctive techniques (ie, chimney grafts) and hybrid combinations continue to gain momentum.
Branched technologies represent one of the next important phases in TEVAR, and a notorious target of ongoing research and development efforts everywhere. In future years, multiple iterations and designs will likely become available (Figure 2). In the interim, clinical application of various adjunctive techniques (ie, chimney grafts) and hybrid combinations continue to gain momentum.
Aortic Dissection
 Acute aortic dissection (AD) constitutes the most common aortic catastrophe, accounting for more deaths annually than ruptured AAAs. The U.S. incidence is estimated to be 10-15 cases per 100,000 adults/year or approximately 10,000-12,000 new cases/year.22 The frequently malignant clinical course of AD supports the notion of its designation as a major cardiovascular entity despite the relatively low incidence. An intimomedial tear (entry tear) occurs first, allowing the torrential aortic blood flow to rip into the wall, creating a secondary flow channel, or false lumen (FL), that propagates distally in a spiraled (more commonly) or straight fashion to involve various lengths in the aorta, not infrequently all the way down to the bifurcation and into one or both iliac arteries. The FL can also propagate proximally. The FL is highly pressurized and often compresses the true lumen in the chest, which can result in virtual collapse and impediment of distal aortic blood flow (malperfusion) to kidneys and viscera, the lower extremities, and/or the spinal cord. The entry tear is usually located in the thoracic aorta: ascending aorta for type A and descending just distal to the origin of the left subclavian artery in type B. Secondary or reentry tears (spontaneous fenestrations) can and frequently occur in the distal thoracic and/or the abdominal aorta.
Acute aortic dissection (AD) constitutes the most common aortic catastrophe, accounting for more deaths annually than ruptured AAAs. The U.S. incidence is estimated to be 10-15 cases per 100,000 adults/year or approximately 10,000-12,000 new cases/year.22 The frequently malignant clinical course of AD supports the notion of its designation as a major cardiovascular entity despite the relatively low incidence. An intimomedial tear (entry tear) occurs first, allowing the torrential aortic blood flow to rip into the wall, creating a secondary flow channel, or false lumen (FL), that propagates distally in a spiraled (more commonly) or straight fashion to involve various lengths in the aorta, not infrequently all the way down to the bifurcation and into one or both iliac arteries. The FL can also propagate proximally. The FL is highly pressurized and often compresses the true lumen in the chest, which can result in virtual collapse and impediment of distal aortic blood flow (malperfusion) to kidneys and viscera, the lower extremities, and/or the spinal cord. The entry tear is usually located in the thoracic aorta: ascending aorta for type A and descending just distal to the origin of the left subclavian artery in type B. Secondary or reentry tears (spontaneous fenestrations) can and frequently occur in the distal thoracic and/or the abdominal aorta.
Weakening of the aortic wall because of medial degeneration is the culprit defect that sets the occurrence of aortic dissection into motion. Inherited connective tissue disorders such as Marfan syndrome (and Ehlers-Danlos or Loeys-Dietz syndromes), and other TAA and aortic dissection familial syndromes represent possible etiologies as well.23 However, the vast majority of AD patients suffer from severe, often uncontrolled or poorly treated, arterial hypertension, which causes severe degenerative changes in the aortic wall over time. It is not clear if hypertension alone can cause aortic dissection without a predisposed or weakened aorta. Diameter plays a role too because aortic dilatation results in increasing wall tension and mechanical stress.11
The International Registry of Acute Aortic Dissection24 has produced impressive information on several key features related to this disease. For instance, the most important risk factors for the development of acute AD would appear to be: male gender (2:1 male-to-female ratio), age (6th and 7th decade), history of hypertension, prior cardiac surgery (including aortic valve replacement or repair), bicuspid aortic valve, Marfan syndrome, and crack cocaine use. Two other disorders, intramural hematoma (IMH) and PAU, tend to present with similar symptoms as dissections and may be linked etiologically and pathogenically. Hemorrhage within the aortic wall without an intimomedial flap or tear causes IMH. It can be a precursor of dissection in many cases and can evolve into a classic AD (with a double lumen) as shown in almost 20% of afflicted patients.25 The majority (2/3) are classified as type B because they involve the descending thoracic aorta. As to PAU, most are located in the descending aorta and often occur in elderly patients with severe generalized atherosclerosis. It can also be a precursor to aortic dissection and is associated with IMH. All 3 conditions with the ruptured TAA are grouped together in the quartet of the acute aortic syndrome.26
Clinical definition of AD recognizes a case as acute when the symptoms are of 14 days duration or less. After the first 2 weeks, AD is labeled to be chronic. Such designations evolved when the vast majority of acute AD patients died within a few hours or days from onset, which may explain the arbitrary use of the term chronic from the 15th day on.27 Anatomically, AD is classified according to extent and location in the aorta:
- DeBakey’s types I and II (or Stanford type A): ascending aortic involvement (plus/minus more distal extension)
- Type IIIa and IIIb (Stanford type B): AD begins beyond the origin of the left subclavian artery and propagates distally for various lengths.27
The mortality rate of untreated acute type A dissection is overwhelming: about 1/3 of patients die within the first 24 hours, and 50% by the end of the second day with an 80% mortality rate at 2 weeks.
The terms complicated and uncomplicated are used to further characterize acute type B (type III). Complicated dissection is diagnosed in the face of severe complications and symptoms such as rupture (blood outside the aortic wall), malperfusion (visceral/renal, spinal cord, and/or the lower extremities), or acute diameter expansion in the distal arch or proximal descending aorta. Unrelenting pain, uncontrolled hypertension, and image worsening are also considered components of this definition. Approximately 30% of acute type B patients are diagnosed as complicated and exposed to grave danger without urgent intervention. The other 70% present with uncomplicated dissections and most are managed medically with pharmacologic anti-impulse, antihypertensive therapy, and pain control. Patients with uncomplicated acute AD treated with modern optimal medical therapy face a 10% 30-day mortality rate. However, they are exposed to possible serious mid- and long-term complications including the development of a dissecting TAA out of the enlarging FL in the chest in 20%-30% of cases.28 Close monitoring and follow-up (with serial imaging) are therefore paramount. AD complicated by overt ischemia (malperfusion) or rupture requires prompt intervention, and patients face a relatively high 20% mortality or more within the first 30 days.29
Open surgical repair continues to carry high morbidity and mortality risks.29 The endovascular approach began in 1999 with the publication of 2 landmark articles in the New England Journal of Medicine.30,31 The pioneering early work of Nienaber et al and Dake et al suggested strongly that endovascular treatment of acute AD using a stent graft device (to cover and close the entry tear) might offer a safer and more attractive alternative to traditional open surgery. Interest in such strategies increased rapidly a few years later, and a rapidly growing clinical experience has largely substantiated their initial observations.32,33
TEVAR today is increasingly considered as first-line therapy for the majority of complicated AD patients presenting with an interventional imperative—a true paradigm shift. However, high-level supporting scientific evidence in favor of this shift is weak and nearly non-existent, and treatment of AD with the thoracic stent graft devices represents off-label use at this time. The situation may change for the better in the foreseeable future as ongoing and new trials provide (hopefully) the necessary clinical evidence to support an on-label indication.34 For uncomplicated AD, there is near consensus that medical treatment is best at present for management of most such patients, at least initially.
Availability of dissection-specific devices is another important goal. Cook Medical pursued this goal before anyone else with development of the TXD stent graft, which recently received CE mark. It consists of a proximal standard endograft that can be extended distally using a variable number of interlinked bare-metal self-expanding stents to stabilize the dissected intima and re-expand the true lumen throughout its extent of the disease (Figure 2). This technique could be applicable with the combination of other available devices as well. Early clinical experiences and patient outcomes are encouraging thusfar.35 Other AD-specific endograft designs will likely follow over the next several years, and they will probably not have proximal bare stents or bare stents that are short, soft, and feature well-rounded smooth peaks.
Traumatic Thoracic Aortic Injury
As thoracic aortic injuries (TAI) usually do not involve a complete loss of continuity across the vessel, the term transection is misleading and inaccurate, but has nonetheless come to signify blunt traumatic aortic thoracic disruptions that are most frequently fatal. Many are related to deceleration injuries occurring during motor vehicle accidents. Nearly 20% of fatalities related to vehicular collisions are caused by TAI,36 and it is the second most frequent cause of traumatic death overall.37 Eighty to 90% of victims die immediately at the accident site. Up to 50% of those who make it to the hospital alive die within 24 hours. Annually, there are as many as 8,000 such injuries in the U.S. and approximately 1,000 victims arrive at the hospital alive.38
Historically, surgical treatment is accompanied by a mortality of 28% on average with a 16% paraplegia rate. Endovascular repair is growing in acceptance and adoption as the results appear to be better than those of open surgery.38-40 In many centers, TEVAR has already replaced surgical treatment in the management of most trauma victims41 and TAI is now an on-label indication for stent graft intervention as Gore’s Conformable TAG device received FDA approval in January 2012. Medtronic’s and Cook’s devices will likely follow suit later in the year.
A new TAI classification that recognizes 4 different extents of aortic injuries is another helpful addition to our body of knowledge and is beginning to improve our ability to make better informed decisions on which lesions to select for repair strategies versus those that may be safely observed without intervention.42 Unfortunately, we still have very little if any information on long-term outcomes after stent graft repair in this indication.
In conclusion, the TEVAR landscape 2012 is one of profound ongoing transformation, paradigm shifts, and intense research and development. The pace of change will likely continue and even accelerate in the foreseeable future. While true that there are still several challenges and unfulfilled promises, it is unequivocal that we have been empowered with the availability of important new tools and a much enhanced ability to treat a large number of complex life-threatening aortic pathologies, providing enormous benefit to many patients.
References
- Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331(26):1729-1734.
- Criado FJ. EVAR at 20: the unfolding of a revolutionary new technique that changed everything. J Endovasc Ther. 2010;17(6):789-796.
- Criado, FJ. The EVAR landscape in 2011: a status report on AAA therapy. Endovascular Today. 2011;40-44,58.
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73(1):17-27.
- Ramanath VS, Oh JK, Sundt TM 3rd, Eagle KA. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc. 2009;84(5):465-481.
- Svensson LG, Kouchoukos NT, Miller DC, et al; for the Society of Thoracic Surgeons Endovascular Surgery Task Force. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(1 Suppl):S1-41.
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74(5):S1877-1880.
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176-180.
- Jonker FH, Trimarchi S, Verhagen HJ, Moll FL, Sumpio BE, Muhs BE. Meta-analysis of open versus endovascular repair for ruptured descending thoracic aortic aneurysm. J Vasc Surg. 2010;51(4):1026-1032.
- Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160(10):1425-1430.
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Yale J Biol Med. 2008;81(4):175-186.
- Elefteriades JA, Rizzo JA. Epidemiology, prevalence, incidence, trends. In: Elefteriades JA, ed. Acute Aortic Disease. New York, NY: Informa Healthcare; 2008:89-98.
- Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 2006;44(2):237-243.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55(9):841-857.
- Lam CR, Aram HH. Resection of the descending thoracic aorta for aneurysm; a report of the use of a homograft in a case and an experimental study. Ann Surg. 1951;134(4):743-752.
- Conrad MF, Cambria RP. Contemporary management of descending thoracic and thoracoabdominal aortic aneurysms: endovascular versus open. Circulation. 2008;117(6):841-852.
- Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP; for the Gore TAG Investigators. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47(5):912-918.
- Greenberg RK, O’Neill S, Walker E, et al. Endovascular repair of thoracic aortic lesions with the Zenith TX1 and TX2 thoracic grafts: intermediate-term results. J Vasc Surg. 2005;41(4):589-596.
- Fairman RM, Criado F, Farber M, et al; for the VALOR Investigators. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008;48(3):546-554.
- Fairman RM. Results of the VALOR II trial of the Medtronic Valiant thoracic stent-graft at 1 year. Presented at the 2011 SVS Vascular Annual Meeting, June 16-28, Chicago, IL.
- Walker KL, Shuster JJ, Martin TD, et al. Practice patterns for thoracic aneurysms in the stent graft era: health care system implications. Ann Thorac Surg. 2010;90(6):1833-1839.
- Svensson LG, Kouchoukos NT, Miller DC, et al; for the Society of Thoracic Surgeons Endovascular Surgery Task Force. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(1 Suppl):S1-41.
- Fattori R, Napoli G, Lovato L, et al. Descending thoracic aortic diseases: stent-graft repair. Radiology. 2003;229(1):176-183.
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897-903.
- Evangelista A, Mukherjee D, Mehta RH, et al; for the International Registry of Aortic Dissection (IRAD) Investigators. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111(8):1063-1070.
- Nienaber CA, Powell JT. Management of acute aortic syndrome. Eur Heart J. 2012;33(1):26-35.
- Criado FJ. The mystery of aortic dissection: a 250-year evolution. J Cardiovasc Surg. 2010;51(5):601-608.
- Grabenwoger M, Fleck T, Czerny M, et al. Endovascular stent graft placement in patients with acute thoracic aortic syndromes. Eur J Cardiothorac Surg. 2003;23(5):788-793.
- Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg. 2008;85(3):965-971.
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340(20):1539-1545.
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340(20):1546-1552.
- Khoynezhad A, Donayre CE, Omari BO, Kopchok GE, Walot I, White RA. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg. 2009;138(3):625-631.
- White RA, Miller DC, Criado FJ, et al; for the Multidisciplinary Society for Vascular Surgery Outcomes Committee. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg. 2011;53(4):1082-1090.
- Investigators for the Medtronic Valiant Captivia Trial. Clinical study to evaluate the performance of the Valiant thoracic stent-graft with the Captivia delivery system (Valiant Captivia) for the treatment of acute, complicated type B aortic dissections. Study in progress.
- Lombardi JV, Nienaber CA, Cambria R, et al. Endovascular treatment of complicated type B aortic dissection using a composite device design: initial results of a prospective multicenter clinical trial (STABLE). Presented at the 2011 SVS Annual Vascular Meeting, June 16-28, Chicago, IL.
- Rousseau H, Soula P, Perreault P, et al. Delayed treatment of traumatic rupture of the thoracic aorta with endoluminal covered stent. Circulation. 1999;99(4):498-504.
- Plummer D, Petro K, Akbari C, O’Donnell S. Endovascular repair of traumatic thoracic aortic disruption. Perspect Vasc Surg Endovasc Ther. 2006;18(2):132-139.
- Neschis DG, Moaine S, Gutta R, et al. Twenty consecutive cases of endograft repair of traumatic aortic disruption: lessons learned. J Vasc Surg. 2007;45(3):487-492.
- Hong MS, Feezor RJ, Lee WA, Nelson PR. The advent of thoracic endovascular aortic repair is associated with broadened treatment eligibility and decreased overall mortality in traumatic thoracic aortic injury. J Vasc Surg. 2011;53(1):36-43.
- Estrera AL, Gochnour DC, Azizzadeh A, et al. Progress in the treatment of blunt thoracic aortic injury: 12-year single-institution experience. Ann Thorac Surg. 2010;90(1):64-71.
- Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187-192.
- Azizzadeh A, Keyhani K, Miller CC 3rd, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovWWascular repair. J Vasc Surg. 2009;49(6):1403-1408.
__________________________________________________
From the MedStar Union Memorial Hospital, Baltimore, Maryland.
Disclosure: The author has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The author reports that he has consulted and developed educational presentations for Medtronic.










