TEVAR Update 2013: Regulatory, Technology, and Therapy Trends
This 2013 TEVAR update represents a follow-up to the 2012 VDM article that summarized thethoracic endovascular landscape.1 Unlike the previous article, the current update has been constructed using a format with only limited text and commentaries. It is our hope that, while becoming an excellent vehicle to highlight all relevant and newsworthy regulatory, technology, and therapy trends and developments, this update will also prove easier to read and emerge perhaps as a more malleable and versatile platform for subsequent offerings of annual or semiannual updates.
United States Regulatory Update
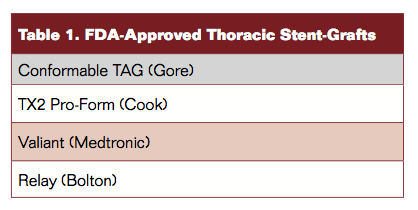
Table 1 lists the 4 currently approved and commercially available thoracic stent-graft devices in the United States (Figure 1). Additional stent-graft choices exist in other countries.
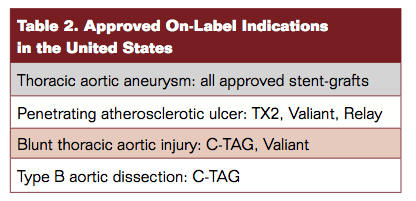
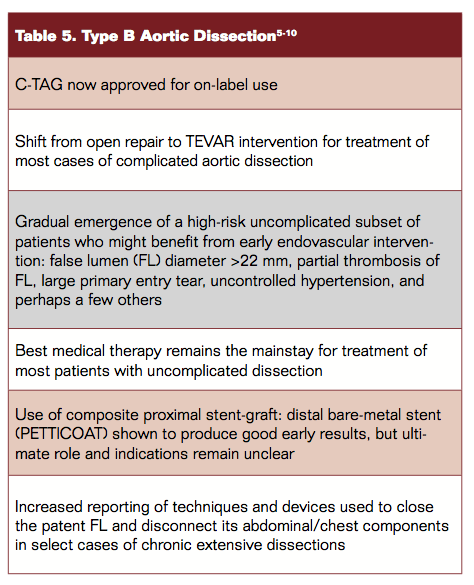
Table 2 depicts the approved on-label indications. Recent approval of the Conformable TAG (C-TAG; Gore) device qualifies easily (in the author’s view) as the most significant development of 2013 so far.
Technology Update
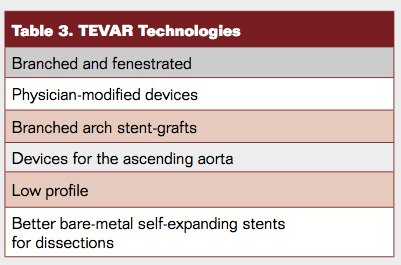
Noteworthy technology updates and evolutions (Table 3) can be summarized as follows:
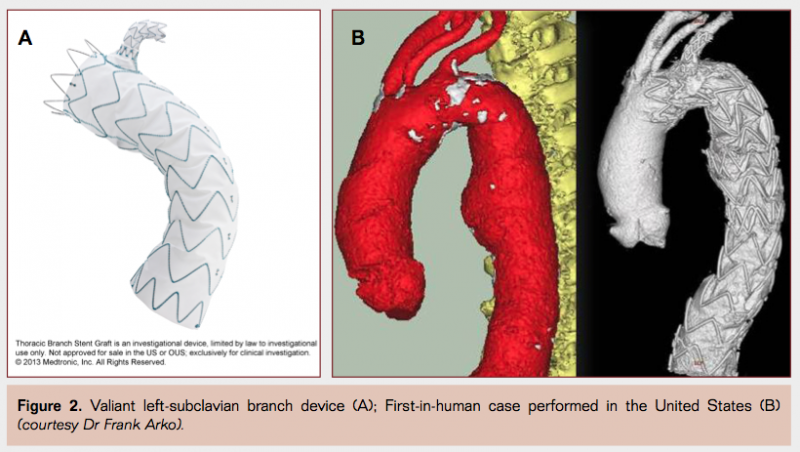
- Branched devices (Figure 2): the Medtronic Mona LSA subclavian-branch Valiant stent-graft project has attracted a great deal of interest because it represents an important innovation (Figure 2A) and because of the unique and brand-new FDA-sanctioned regulatory pathway that enabled the new technology to be channeled through an expedited investigational process and to undergo first-in-human implantation in the United States (Figure 2B). This is a “first” for such a device to be so tested in many years.
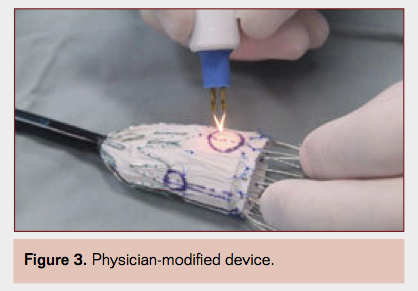
- Physician-modified devices (Figure 3) have received considerable attention in the recent past, including a number of publications from a few centers of excellence where such complex aortic procedures are increasingly performed.2,3 It is reassuring and not surprising that most of these efforts have been supported by an FDA-approved investigational device exemption. No doubt most such developments are largely the result of a lack of availability of branched and fenestrated devices in this country and are likely to cease for the most part as new technologies become more commonplace in the foreseeable future. It is also important to note that modifying a commercial device in such a manner violates the manufacturer’s seal of approval and warranty and carries significant risks and potential pitfalls.
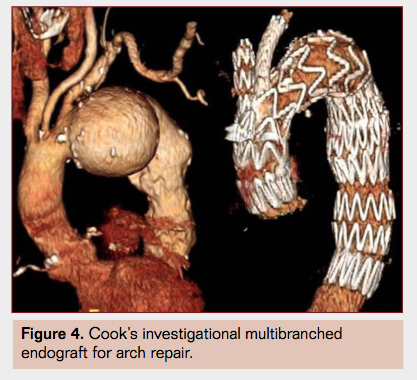
- Arch endograft designs with multiple branches (Figure 4) are being tested but remain experimental and unproven at this time.4
- Ascending aorta designs (Figure 5) are beginning to emerge as anatomical opportunities for endovascular repair of some patients with acute type A dissections are becoming more clear. While much work remains to be done before any of this becomes part of the TEVAR armamentarium, these developments are nonetheless exciting as they offer hope of creating a viable nonsurgical treatment option that may be applicable in a significant minority of such cases.
- Lowering the profile of thoracic stent-grafts has remained an elusive goal through the last wave of technological refinements and new device iterations. But at last, this will change for good as 20 Fr maximum outer diameter delivery system profiles are now all but sure to become a reality and, more importantly, available in the near future.
- Lastly, softer and gentler bare-metal stents (Figure 6) are likely to become available soon for use as a composite combination with a proximal thoracic stent-graft (PETTICOAT: provisional extension to induce complete attachment) in the treatment of extensive type B dissections.

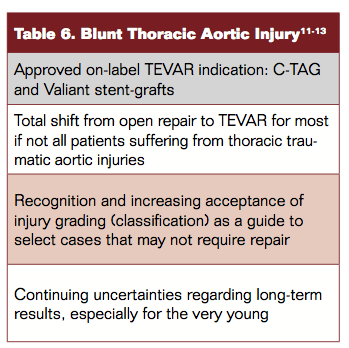
TEVAR Therapy Trends
Tables 4 to 6 depict trends in TEVAR therapies. 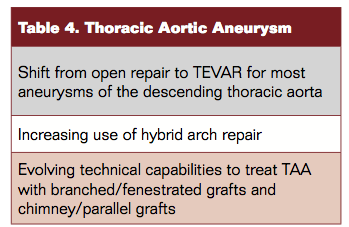
Finally, Table 7 summarizes briefly the most significant unmet needs as they remain unresolved in the 2013 TEVAR landscape.

Editor’s Note: Disclosure: The author has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Criado reports consultancy to and honoraria and reimbursements from Medtronic.
Address for correspondence: Frank J. Criado, MD, FACS, FSVM, MedStar Union Memorial Hospital, 3333 N. Calvert St. Suite 560, Baltimore, Maryland 21218, USA.
References
- Criado FJ. Thoracic aortic endografting: status report 2012. Vasc Dis Manag.2012;9(7):E125-E130.
- Oderich GS, Ricotta JJ 2nd. Modified fenestrated stent grafts: device design, modifications, implantation, and current applications. Perspect Vasc Surg Endovasc Ther. 2009;21(3):157-167.
- Ricotta JJ II, Tsilimparis N. Surgeon-modified fenestrated and branched stent grafts. Endovasc Today. 2011;November:55-60.
- Lioupis C, Corriveau MM, MacKenzie KS, Obrand DI, Steinmetz OK, Abraham CZ. Treatment of aortic arch aneurysms with a modular transfemoral multibranched stent graft: initial experience. Eur J Vasc Endovasc Surg. 2012;43(5):525-532.
- Nienaber CA, Kische S, Rousseau H, et al. for the INSTEAD XL trial. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6(4):407-416.
- Thrumurthy SG, Karthikesalingam K, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42(5):632-647.
- Kitagawa A, Greenberg RK, Eagleton MJ, Mastracci TM, Roselli EE. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection withthoracoabdominal aneurysms. J Vasc Surg. 2013;58(3):625-634.
- Lombardi JV, Cambria RP, Nienaber CA et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55(3):629-640.
- Merola J, Garg K, Adelman MA, et al. Endovascular versus medical therapy for uncomplicated type B aortic dissection: a qualitative review. Vasc and Endovasc Surg. 2013;47(7):497-501.
- Kölbel T, Lohrenz C, Kieback A, Diener H, Debus ES, Larena-Avellaneda A. Distal falselumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther. 2013;20(4):484-489.
- Garcia-Toca M, Naughton PA, Matsumura JS, et al. Endovascular repair of blunt traumatic thoracic aortic injuries: seven-year single-center experience. Arch Surg. 2010;145(7):679-683.
- Azizzadeh A, Keyhani K, Miller CC III, et al. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49(6):1403-1408.
- Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187-192.