Paclitaxel Townhall: Is There Enough Concern to Alter Current Standards of Practice?
The ISET 2019 Townhall on paclitaxel addressed the controversy surrounding a recent meta-analysis by Katsanos and colleagues that has potentially significant implications for changing practice in the field. Barry Katzen, MD, introduced the topic and explained that ISET will explore the issue and leave participants with a sense of the opinion of the audience.
The session began with Juan Granada, MD, taking the stage to provide insight into the pharmacology and mechanism of action of paclitaxel. “The pharmacokinetics and pharmacodynamics of paclitaxel in humans are well-characterized,” he said. Paclitaxel has hepatic clearance and can produce a range of toxicities, including bone marrow suppression (primarily neurotropenia), though this is dose dependent and only happens with multiple infusions of the drug, said Dr. Granada.
He went on to describe the differences in DCB use and intravenous infusions of paclitaxel. “The typical concentration of DCB, maximal exposure is way below that of IV use of paclitaxel,” he said.
He explained that paclitaxel pharmacokinetics following use of DCBs are unique, non-linear, and dependent on the solubility profile of the coating. When DCB at its highest dose is compared with systemic use, DCB use still results in lower systemic exposure and transient multi-organ distribution and concentration. “Systemic tissue concentrations remain below the therapeutic threshold and are unlikely to exert a sustained biological effect, especially at 5 years,” said Dr. Granada.
The next speaker, Jihad Mustapha, MD, reviewed the data in the manuscript and offered commentary and analysis from his perspective. Katsanos and colleagues report increased risk of death at 2 years and beyond with paclitaxel-coated dives for treatment of femoropopliteal arteries. “The authors raised concern that a therapy commonly perceived as safe may actually increase mortality risk,” he explained.
Dr. Mustapha pointed out that in the Katsanos study, mortality rates for each treatment group were calculated as total number of observed deaths divided by a total number of enrolled patients. Metrics such as the number of withdrawals, the number of patients remaining at risk, and the timing of deaths were not considered in the study’s calculations.
He illustrated the implications of analysis choices by the authors. For example, there may be discrepancies between crude versus time-to-event mortality rates among other studies in the meta-analysis, especially those with longer follow-up periods during which higher rates of patient withdrawal might be expected.
“It would be interesting to reanalyze the meta-analysis findings using mortality rates derived from time-to-event methods to assess whether the conclusion of increased mortality risk with paclitaxel-coated devices is confirmed or refuted,” said Dr. Mustapha. He added that further exploration of the potential mechanisms of paclitaxel contributing to excess mortality should also be explored, as causes of late deaths were mostly unreported or considered unrelated to the device or procedure.
Thomas Zeller, MD, continued the session with an update on the European response to the Katsanos findings. Dr. Zeller had been involved in many of the studies incorporated in the Katsanos analysis and offered his insights into the methods used in the meta-analysis.
Dr. Zeller has responded to Dr. Katsanos in a recently published editorial in which he pointed out that the Katsanos study classified DCB and DES as a single homogenous device class even though they substantially differ in features such as dose, release kinetics, and performance. Additionally, the meta-analysis does not have patient-level data.
“In my opinion, [the Katsanos study] is no reason to panic,” he said.
He also updated the audience on the conversation at LINC, in which the major companies released additional data regarding safety. The data showed that none of the devices discussed showed a clear, significant correlation with mortality.
Dr. Zeller concluded by affirming that paclitaxel is a toxic drug and that local vessel wall damage is reported and not yet understood in detail. “Systemic toxicity with low-dose paclitaxel release via DCB or DES seems to be unlikely but warrants further observation,” he said.
Gary Ansel, MD, presented next, speaking about long-term data from IN.PACT DCB and Zilver PTX programs. He explained that the findings of the meta-analysis cause concern, as none of the trials previously done have been powered for mortality after 1 year. “If done properly, a meta-analysis will bring to light potential risks or concerns, or improvements, over the years compared to individual trials,” he said.
After reviewing data from IN.PACT and other studies, he shared his thoughts. “Are drug-coated devices safe? We don’t know that yet, but looking at the data give us reasonable comfort. We await the VIVA leaders forum and the blinded combined industry data analysis for further analysis and guidance.”
Michael Jaff, DO, was the final speaker. He offered the perspective of a vascular specialist who often refers patients to procedures involving DCBs and paclitaxel. He described his surprise at learning about the study’s conclusions via a phone alert just before seeing a patient who was seeking his opinion on whether undergo a DCB procedure. Dr. Jaff ultimately told the patient that he is monitoring concerns about a recent study and that he will better inform the patient of his opinion at the next visit.
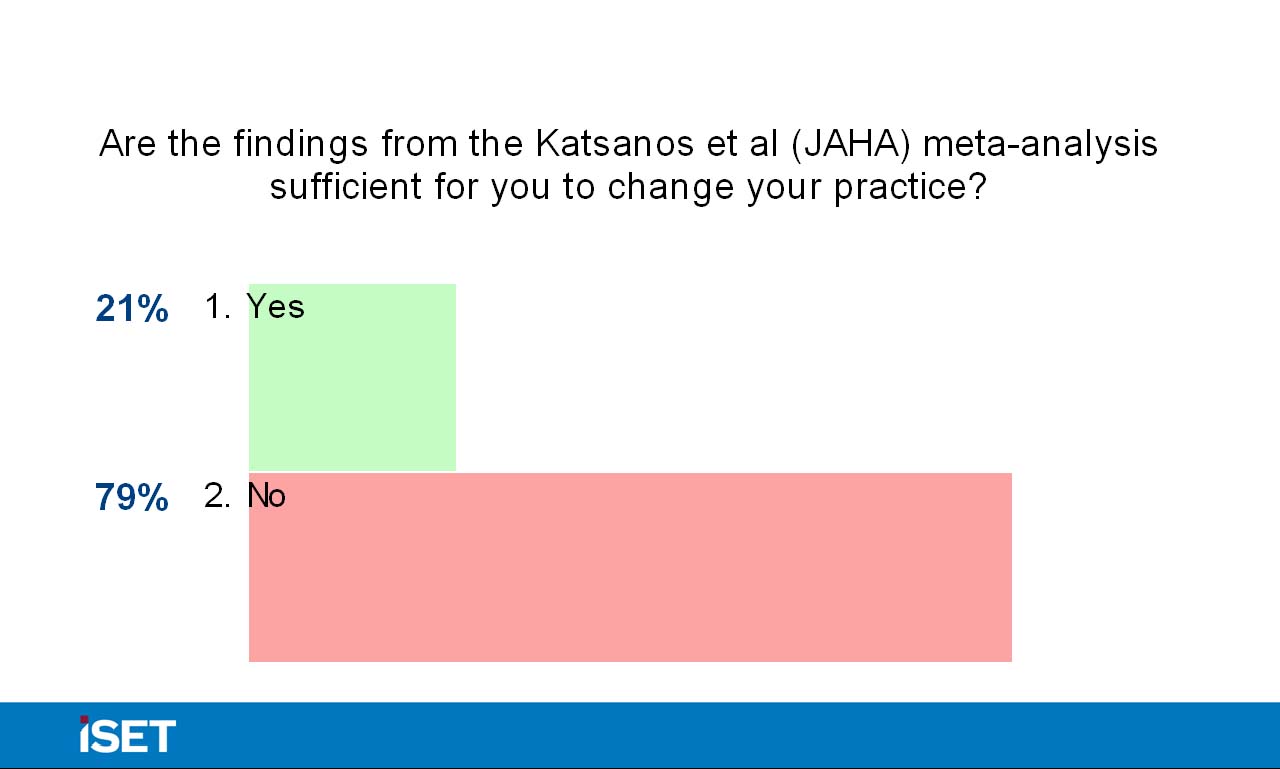
Dr. Katzen then returned the discussion to the panel and asked them whether they have personally changed their practice. Dr. Ansel’s institution has left the decision up the individual practitioner and has not created a uniform suggestion. Panel members’ responses varied in terms of discussion with patients, with some opting to proactively broach the topic and others mentioning the study if prompted by the patient. Dr. Katzen also engaged the audience in the debate via interactive polling, and 79% of the audience responded that they are not currently changing their use of drug-eluting technologies based on the findings of the meta-analysis. Additionally, nearly 70% of the audience members did not believe the suggestion of mortality as defined in the Katsanos paper was statistically valid, and 84% of audience members did not believe that current trials should be halted based on the study results. Dr. Katzen concluded the session by thanking the panelists and audience for their participation, and he assured them that the discussion about paclitaxel will continue throughout the conference. 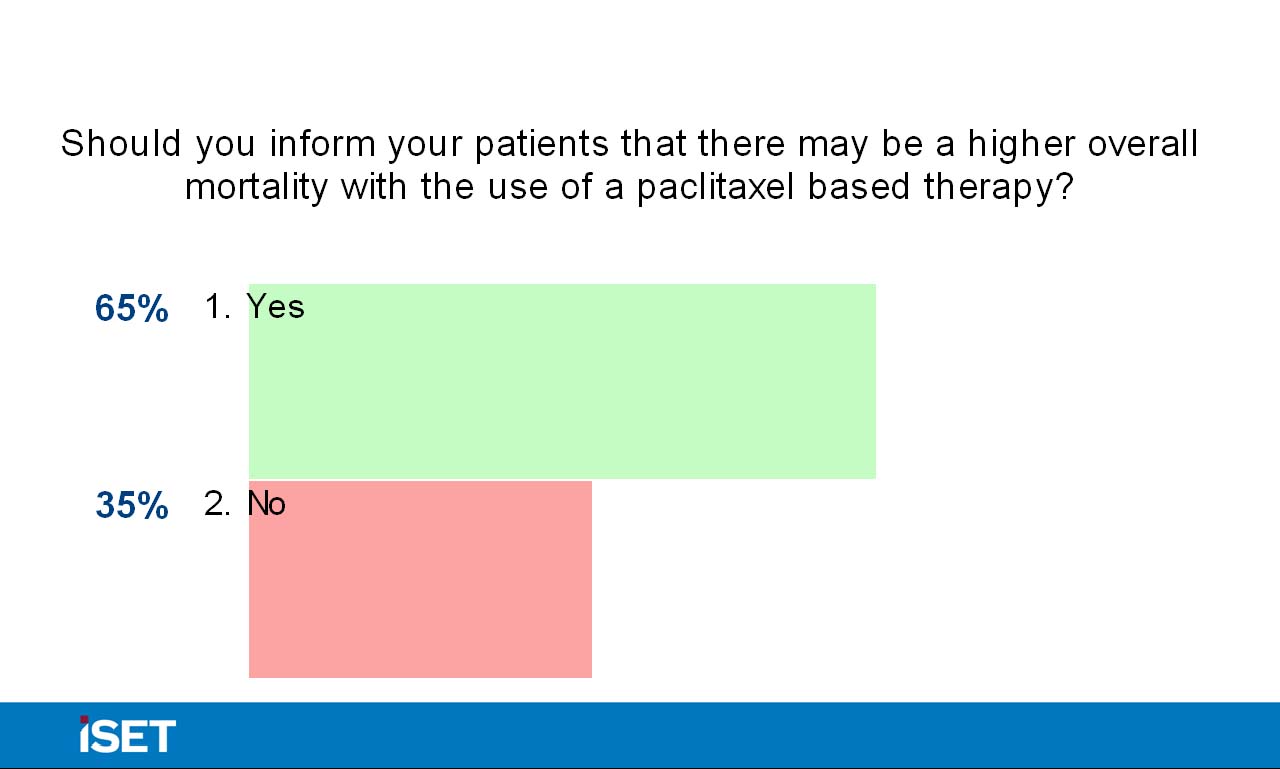

—Lauren LeBano













