Embolization of a Chest Wall Arterial Venous Malformation Using a Transradial Approach
ABSTRACT: We report the case of an 18-year-old male with a peripheral arteriovenous malformation (AVM) of the chest wall treated with embolization using a transradial approach (TRA). Chest wall AVMs are exceedingly rare, with very few case reports published. Treatment options are varied and include monitoring, surgical resection, and endovascular embolization. While less invasive, endovascular management can be complicated by the ability to both identify and cannulate arterial “feeder” vessels. Compared to a transfemoral approach, a TRA has been shown to reduce access-site complications and bleeding. However, technical factors such as differences in patient-specific vascular anatomy and catheter length required for intervention can also weigh heavily in access-site determination. We report on the usefulness of TRA in embolization of a chest wall AVM with inferior coursing, intercostal “feeder” arteries forming obtuse angles with the descending thoracic aorta, more readily cannulated by a superior TRA.
VASCULAR DISEASE MANAGEMENT 2016;13(10):E230-E235
Key words: arteriovenous malformation, transradial catheterization, embolization
_____________________________________________________________
Arteriovenous malformations (AVMs) are anatomically abnormal connections between the arterial and venous systems. While most reported cases of chest wall AVMs are caused by acquired etiologies, including trauma and infection, they can also be caused by congenital etiologies as seen in hereditary hemorrhagic telangiectasia or neurofibromatosis type 1.1-7 The majority of AVMs are asymptomatic, yet a certain percentage can cause high-output cardiac failure, intense pain, and major bleeding. Appropriate management includes monitoring, sclerotherapy, laser, endovascular embolization, and surgical resection.8
Case Report
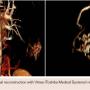
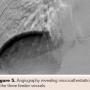
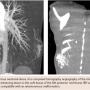 An 18-year old male presented with a chief complaint of an intermittently painful lump on the right posterior thorax, which had been progressively enlarging over the past month. He denied fever, weight loss, or trauma tobaffected area. On physical exam, a 3 cm mobile/pulsatile mass at the right flank/posterior chest was visualized. His lungs were clear to auscultation and his heart had a regular rate and rhythm. The patient had no other past medical history. The patient was admitted to our hospital for further work-up. The differential diagnosis upon admission included lipoma, lymphoma, osteosarcoma, and arteriovenous malformation. Laboratory values of complete blood count and basic metabolic profile were within reference range. Computed tomography (CT) angiography of the chest with intravenous (IV) contrast revealed an enhancing lesion in the soft tissue of the posterior mid thorax (Figure 1) with rapid arterial filling and venous wash-out compatible with an AVM. Three-dimensional reconstruction with Vitrea software (Toshiba Medical Systems) revealed 3 arterial intercostal feeders (Figure 2). The posterior chest wall AVM blood supply was visualized on CT chest with IV contrast as deriving from three intercostal feeder arteries (Figure 3). Based on current management guidelines, peripheral AVMs are generally treated conservatively unless persistent pain is present. Our patient complained of intermittent pain hence we planned for endovascular embolization. After obtaining informed consent, peripheral embolization of the chest wall AVM was performed.
An 18-year old male presented with a chief complaint of an intermittently painful lump on the right posterior thorax, which had been progressively enlarging over the past month. He denied fever, weight loss, or trauma tobaffected area. On physical exam, a 3 cm mobile/pulsatile mass at the right flank/posterior chest was visualized. His lungs were clear to auscultation and his heart had a regular rate and rhythm. The patient had no other past medical history. The patient was admitted to our hospital for further work-up. The differential diagnosis upon admission included lipoma, lymphoma, osteosarcoma, and arteriovenous malformation. Laboratory values of complete blood count and basic metabolic profile were within reference range. Computed tomography (CT) angiography of the chest with intravenous (IV) contrast revealed an enhancing lesion in the soft tissue of the posterior mid thorax (Figure 1) with rapid arterial filling and venous wash-out compatible with an AVM. Three-dimensional reconstruction with Vitrea software (Toshiba Medical Systems) revealed 3 arterial intercostal feeders (Figure 2). The posterior chest wall AVM blood supply was visualized on CT chest with IV contrast as deriving from three intercostal feeder arteries (Figure 3). Based on current management guidelines, peripheral AVMs are generally treated conservatively unless persistent pain is present. Our patient complained of intermittent pain hence we planned for endovascular embolization. After obtaining informed consent, peripheral embolization of the chest wall AVM was performed.
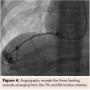
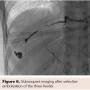
 To confirm dual circulation to the left hand, a Barbeau test was performed, which exhibited a type B response and hence appropriate for transradial catheterization of the left wrist. Access of the left radial artery was gained using a 21 gauge micropuncture needle. A 6 Fr hydrophilic Glidesheath Slender (Terumo Interventional Systems) was introduced with subsequent injection of 3,000 units of heparin, 200 micrograms of nitroglycerin, and 2.5 mg of verapamil. A 5 Fr 110 cm Optitorque pigtail catheter (Terumo Interventional Systems) was introduced into the descending aorta. Angiograghy was performed revealing three feeding vessels off the 7th and 8th lumbar arteries (Figure 4). A 6 Fr 110 cm RunWay Guide Catheter JR4 curve (Boston Scientific) was used to select each feeding artery. Subsequently, a Renegade Hi-Flo Microcatheter with a 10 cm distal tip and a 180 cm Fathom-16 steerable guidewire (Boston Scientific) were advanced into each of the three feeder vessels (Figure 5). Onyx 34 liquid embolic system (Covidien) was introduced into each feeder vessel for embolization (Figure 6). Embolization was performed until adequate stasis was achieved. No evidence of residual arterial enhancement was seen on the postembolization angiogram.
To confirm dual circulation to the left hand, a Barbeau test was performed, which exhibited a type B response and hence appropriate for transradial catheterization of the left wrist. Access of the left radial artery was gained using a 21 gauge micropuncture needle. A 6 Fr hydrophilic Glidesheath Slender (Terumo Interventional Systems) was introduced with subsequent injection of 3,000 units of heparin, 200 micrograms of nitroglycerin, and 2.5 mg of verapamil. A 5 Fr 110 cm Optitorque pigtail catheter (Terumo Interventional Systems) was introduced into the descending aorta. Angiograghy was performed revealing three feeding vessels off the 7th and 8th lumbar arteries (Figure 4). A 6 Fr 110 cm RunWay Guide Catheter JR4 curve (Boston Scientific) was used to select each feeding artery. Subsequently, a Renegade Hi-Flo Microcatheter with a 10 cm distal tip and a 180 cm Fathom-16 steerable guidewire (Boston Scientific) were advanced into each of the three feeder vessels (Figure 5). Onyx 34 liquid embolic system (Covidien) was introduced into each feeder vessel for embolization (Figure 6). Embolization was performed until adequate stasis was achieved. No evidence of residual arterial enhancement was seen on the postembolization angiogram.
 At completion, a 24 cm TR Band (Terumo Interventional Systems) was placed for patent hemostasis and removed 1 hour later. The postprocedure course was uneventful, no adverse events were observed, and the patient was discharged. In a 3-month follow-up, the patient remained asymptomatic and the mass was no longer palpable. Given clinically asymptomatic state and young age, no follow-up imaging was performed.
At completion, a 24 cm TR Band (Terumo Interventional Systems) was placed for patent hemostasis and removed 1 hour later. The postprocedure course was uneventful, no adverse events were observed, and the patient was discharged. In a 3-month follow-up, the patient remained asymptomatic and the mass was no longer palpable. Given clinically asymptomatic state and young age, no follow-up imaging was performed.
Discussion
 Chest-wall AVMs are rare entities that often do not require clinical intervention. When symptoms such as pain or discomfort are present, conservative management fails and clinical intervention likely in the form of a surgical or endovascular approach is necessary. The major benefit of endovascular treatment includes minimal bleeding, infection risk, and avoidance of an open surgical procedure.
Chest-wall AVMs are rare entities that often do not require clinical intervention. When symptoms such as pain or discomfort are present, conservative management fails and clinical intervention likely in the form of a surgical or endovascular approach is necessary. The major benefit of endovascular treatment includes minimal bleeding, infection risk, and avoidance of an open surgical procedure.
Typically, peripheral vascular interventions are performed via transfemoral access (TFA). The use of the radial artery as the primary access vessel for transcatheter intervention is well known in interventional cardiology, having been described by Campeau at the Montreal Heart Institute as early as 1989, when he suggested the transradial approach as a safer alternative to percutaneous and “cut-down” brachial or axillary access.9 More recent studies from interventional cardiology have shown transradial access (TRA) to have significantly fewer access site and bleeding complications than TFA.10-11 Additionally, benefits in approach angles have been suggested.12
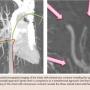
 In this case, TRA provided multiple advantages, which include proximity of the access vessel to the end-target vessels and a better angle for the catheter to approach the end-target vessels. The transradial approach provides a smoother obtuse angle to the chest wall AVM’s feeder vessels while the standard femoral approach provides a more challenging acute angle approach (Figure 3A). The technical success in this particular case showcases that the need for selective microcatheterization in complex procedures does not preclude a radial approach. Other benefits to the transradial approach include fewer vascular complications, lower rates of access bleeding, greater patient satisfaction, immediate ambulation, and procedure cost savings.13-16 Given its significant benefits, TRA has had unique roles in an eclectic variety of embolization procedures including hepatic arterial embolization, uterine artery fibroid embolization, and alternative approach for coiling internal iliac artery prior to endovascular aneurysm exclusion.
In this case, TRA provided multiple advantages, which include proximity of the access vessel to the end-target vessels and a better angle for the catheter to approach the end-target vessels. The transradial approach provides a smoother obtuse angle to the chest wall AVM’s feeder vessels while the standard femoral approach provides a more challenging acute angle approach (Figure 3A). The technical success in this particular case showcases that the need for selective microcatheterization in complex procedures does not preclude a radial approach. Other benefits to the transradial approach include fewer vascular complications, lower rates of access bleeding, greater patient satisfaction, immediate ambulation, and procedure cost savings.13-16 Given its significant benefits, TRA has had unique roles in an eclectic variety of embolization procedures including hepatic arterial embolization, uterine artery fibroid embolization, and alternative approach for coiling internal iliac artery prior to endovascular aneurysm exclusion.
 The selection of embolic agent is also a choice made selectively depending on the clinical setting. Embolic agents vary from coils to plugs to glue to gel foam. We chose to proceed with a liquid embolic to increase the likelihood of distal embolization into the nidus of the AVM. This treatment option has been described and has shown to be clinically successful.17 The specific embolic glue used, Onyx, is a nonadhesive liquid embolic widely used in the treatment of cerebral AVMs with good results.18
The selection of embolic agent is also a choice made selectively depending on the clinical setting. Embolic agents vary from coils to plugs to glue to gel foam. We chose to proceed with a liquid embolic to increase the likelihood of distal embolization into the nidus of the AVM. This treatment option has been described and has shown to be clinically successful.17 The specific embolic glue used, Onyx, is a nonadhesive liquid embolic widely used in the treatment of cerebral AVMs with good results.18
To the best of our knowledge, this is the first case report of transradial embolization of a chest-wall AVM. Our case supports the usefulness, safety, and feasibility of this technique. Hence, transradial approach is a viable option for complicated peripheral endovascular interventions.
Conclusion
We describe a chest-wall AVM successfully treated using a liquid embolic, through a transradial approach. We believe this case highlights the potential value of incorporating decisions regarding access-site selection into any potential endovascular treatment strategy.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Fischman reports receiving fees from Teromu. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript received April 1, 2016; provisional acceptance given June 23, 2016; manuscript accepted July 19, 2016.
Address for correspondence: Safet Lekperic, MD, Department of Radiology, Mount Sinai Hospital, 1 Gustave L. Levy Place, New York, NY 10029, United States. Email: safet.lekperic@mountsinai.org.
References
- Coulter TD, Maurer JR, Miller MT, Mehta AC. Chest wall arteriovenous fistula: an unusual complication after chest tube placement. Ann Thorac Surg. 1999;67(3):849-850.
- Derdeyn CP, Middleton WD, Allen BT, Nordlicht SM. Acquired intercostal arteriovenous fistula: color Doppler ultrasonographic diagnosis. J Ultrasound Med. 1993;12(11):679-681.
- Lai JH, Yan HC, Kao SJ, Lee SC, Shen CY. Intercostal arteriovenous fistula due to pleural biopsy. Thorax. 1990;45(12):976-978.
- Rivera PP, Kole MK, Pelz DM, Gulka IB, McKenzie FN, Lownie SP. Congenital intercostal arteriovenous malformation. AJR Am J Roentgenol. 2006;187(5):W503-W506.
- Saito A, Takahashi T, Ezura M, Tominaga T. Intercostal arteriovenous fistula associated with neurofibromatosis manifesting as congestive myelopathy: case report. Neurosurgery. 2007;61(3):E656-657; discussion E657.
- Yamasaki N, Hata H, Kusaga M, Kubo K. A surgical case of congenital intercostal arteriovenous fistula [in Japanese]. Nihon Kyobu Geka Gakkai Zasshi. 1977;25(7):936-939.
- Yilmaz S, Atinkaya C, Aktas A, Peynircioglu B. Giant arteriovenous malformation located on the chest wall -diagnosis and endovascular treatment: report of a case. Surg Today. 2010;40(12):1164-1168.
- Lee BB, Bergan J, Gloviczki P, et al. Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP)-2009. Int Angiol. 2009;28(6):434-451.
- Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16(1):3-7.
- Bertrand OF, Rao SV, Pancholy S, et al. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3(10):1022-1031.
- Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55(20):2187-2195.
- Raghu C, Louvard Y. Transradial approach for percutaneous transluminal angioplasty and stenting in the treatment of chronic mesenteric ischemia. Catheter Cardiovasc Interv. 2004;61(4):450-454.
- Bertrand OF, Belisle P, Joyal D, et al. Comparison of transradial and femoral approaches for percutaneous coronary interventions: a systematic review and hierarchical Bayesian meta-analysis. Am Heart J. 2012;163(4):632-648.
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60(24):2481-2489.
- Mehta SR, Jolly SS, Cairns J, et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without st-segment elevation. J Am Coll Cardiol. 2012;60(24):2490-2499.
- Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999;138(3 Pt 1):430-436.
- Jahan R, Murayama Y, Gobin YP, Duckwiler GR, Vinters HV, Viñuela F. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48(5):984-995; discussion 995-987.
- Siddhartha W, Parmar H, Shrivastav M, Limaye U. Endovascular glue embolisation of intercostal arteriovenous fistula: a non-surgical treatment option. J Postgrad Med. 2000;46(3):213-214.











