Aorta in Takayasu's Arteritis is as Brittle as Glass: A Case Report
Abstract
Balloon angioplasty of a critically or completely occluded segment of the aorta in Takayasu’s arteritis is challenging because of extensive panarteritis and diffuse fibrosis. In contrast to atheromatous disease, the aorta is left with very little elastic tissue, leading to higher incidence of dissection during intervention. Neither the profile of the angioplasty balloon (compliant vs non-compliant, length, diameter) nor the stent type (covered vs self-expanding) have been defined in performing angioplasty in this situation. We report the case of a 38-year-old female with aortoarteritis. The diseased aorta had diffuse narrowing in its thoracoabdominal part with critical stenosis at the level of the 11th thoracic vertebra. The stenotic segment suffered full-length dissection after balloon dilatation. A self-expanding stent was deployed to contain the dissection. At 12-month follow-up exam, the dissection was healed, without significant lumen loss.
VASCULAR DISEASE MANAGEMENT 2017;14(5):E123-E126
Key words: Takayasu’s arteritis; thoracoabdominal aorta; balloon aortoplasty; dissection
_____________________________________________________________
The diseased aorta in Takayasu’s arteritis features areas of ectasia and stenosis; the aorta is left with very little elastic tissue due to diffuse fibrosis of the intima, media, and externa.1,2 Stent-supported endovascular aortoplasty and surgical revascularization are associated with higher complication and recurrence rates,3,4 even with regular immunomodulation follow-up.5 Unlike atheromatous disease, the inflexible artery6 is vulnerable to dissection, and is as “brittle as glass” during balloon angioplasty.7 Therefore, some authors suggest self-expanding stent or stent-graft supported angioplasty for this condition, with high-pressure dilation8,9 to overcome fibrosis caused by panarteritis. However, the incidence of dissection in this situation appears to be under-reported.
Case report
A 38-year-old female with known Takayasu’s arteritis was referred to us for uncontrollable hypertension because of middle aortic syndrome. She was under regular treatment with immunosuppression. On examination, she had abdominal bruit and weak femoral pulses. Her blood pressure was 210/110 mm Hg in both arms and 120/70 mm Hg in the lower limbs in supine position. Investigations showed inactive phase of disease as suggested by normal level of erythrocyte sedimentation rate and C-reactive protein. The pull-back gradient across the distal thoracic aorta revealed a significant gradient of 110 mm Hg (Figure 1).

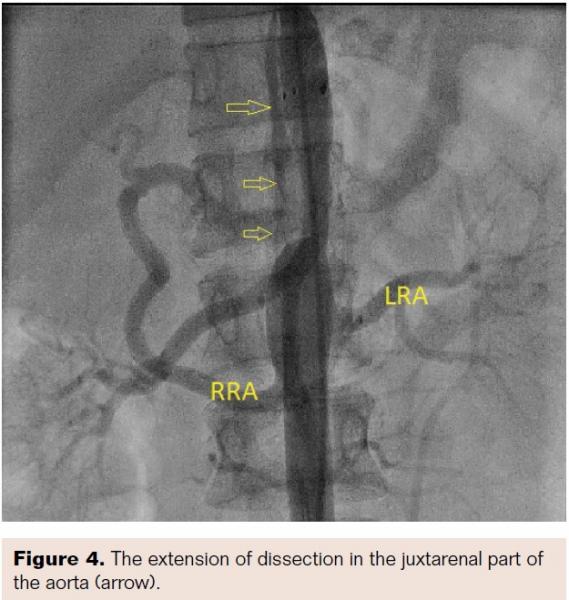
 Aortogram confirmed areas of diffuse stenosis and ectasia in the thoracoabdominal aorta, with significant stenosis of the aorta at the level of intervertebral disc between T-11 and T-12 (Figure 2, Video 1). Informed consent for aortoplasty to control secondary hypertension was obtained. Using right femoral arterial access, the occluded segment was crossed using a straight-tip, exchange-length wire (Terumo) and 5 Fr multipurpose diagnostic catheter. The aortic lesion was dilated using an 8 mm x 4 cm Ravel balloon (Bard Peripheral Vascular) at 4 atm; this size was chosen because the narrowest lesion diameter was 5.2 mm (balloon to lesion ratio of 150%). The patient had mild back pain during and after dilation. The pull-back gradient reduced to 50 mm Hg. A dose of 100 mg intravenous tramadol was given. Immediately, a 12 mm x 6 cm E•Luminex self-expanding stent (Bard Peripheral Vascular) was deployed, as we had no covered stent available at the time of intervention. The pull-back gradient reduced further to less than 20 mm Hg. Repeat aortogram by pigtail catheter revealed extensive dissection of the aorta beyond the disease segment (Figures 3 and 4; Videos 2 and 3). No further dilation was attempted. The patient continued to have mild back pain. Beta-blocker was further reinforced by increasing the dose of atenolol to 100 mg twice daily. Her further hospital course was uneventful. At 12-month follow-up exam, the patient continued to be asymptomatic and her hypertension was under control.
Aortogram confirmed areas of diffuse stenosis and ectasia in the thoracoabdominal aorta, with significant stenosis of the aorta at the level of intervertebral disc between T-11 and T-12 (Figure 2, Video 1). Informed consent for aortoplasty to control secondary hypertension was obtained. Using right femoral arterial access, the occluded segment was crossed using a straight-tip, exchange-length wire (Terumo) and 5 Fr multipurpose diagnostic catheter. The aortic lesion was dilated using an 8 mm x 4 cm Ravel balloon (Bard Peripheral Vascular) at 4 atm; this size was chosen because the narrowest lesion diameter was 5.2 mm (balloon to lesion ratio of 150%). The patient had mild back pain during and after dilation. The pull-back gradient reduced to 50 mm Hg. A dose of 100 mg intravenous tramadol was given. Immediately, a 12 mm x 6 cm E•Luminex self-expanding stent (Bard Peripheral Vascular) was deployed, as we had no covered stent available at the time of intervention. The pull-back gradient reduced further to less than 20 mm Hg. Repeat aortogram by pigtail catheter revealed extensive dissection of the aorta beyond the disease segment (Figures 3 and 4; Videos 2 and 3). No further dilation was attempted. The patient continued to have mild back pain. Beta-blocker was further reinforced by increasing the dose of atenolol to 100 mg twice daily. Her further hospital course was uneventful. At 12-month follow-up exam, the patient continued to be asymptomatic and her hypertension was under control.

Discussion
Angioplasty was first performed for aortoarteritis by Yagura et al in 1984.10 The literature is now robust with a learning curve of more than three decades. It is generally considered that aortoplasty is safe and effective for aortoarteritis, with a low incidence of aortic rupture (which is associated with high mortality).8 We feel the true incidence of aortic dissection or rupture after aortoplasty in Takayasu’s arteritis is under-reported because the studies reporting dissection are available mostly in the form of case reports.11-13 The inelastic vessel wall is tough, non-compliant, and rigid because of panarteritis, and healing occurs by extensive fibrosis. It requires prolonged and repeated balloon dilation at a higher pressure as compared with congenital coarctation or atherosclerosis.14 Balloon angioplasty without a stent in Takayasu’s arteritis is a double-edged sword in that dissection can be the cost of overdilation. However, underdilation invites faster restenosis. The ideal profile of the balloon and the optimum dilation (lesion to reference diameter) are unknown for this condition. The recommendations for balloon sizes in different interventional procedures have been based on guidelines or larger experiences (Table 1). However, we could not find proper answers for the balloon type (compliant vs non-compliant), balloon diameter, balloon length (for diffusely narrowed aorta with some areas of critical stenosis as in our case), or staging of the procedure. We feel it is better to limit the length of balloon to the length of critically stenosed segment. Therefore, it is extremely important to keep a stent-graft option on the shelf before proceeding with aortoplasty. It is also better to inform the on-site surgical team before the procedure. Balloon oversizing, higher inflation pressure, balloon rupture, and wire manipulation have all been implicated in the possible mechanisms of aortic rupture. Accurate sizing of the stent-graft is important because postdilation would further add to the dissection in the face of a lesion that included diffuse stenosis with aneurysmal segments in between.

Conclusion
Aortic dissection is an inherent risk of aortoplasty for patients with Takayasu’s arteritis. We suggest limiting the length of balloon to the critically stenosed segment and a staged procedure when the aorta is diffusely involved with areas of stenosis and aneurysm. Ensure that a covered stent is available for cases of complex lesions. We hope the proper definition of the balloon used for aortoplasty in Takayasu’s arteritis with respect to nature (compliant or non-compliant), size (length and diameter), and inflation pressure will be elucidated by future observations.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted January 6, 2017, provisional acceptance given February 28, 2017, final version accepted March 19, 2017.
Address for correspondence: Ramachandra Barik, DNB (Cardiology), Associate Professor, Department of Cardiology, Nizam’s Institute of Medical Sciences, Hyderabad-500082, Telengana, India. Email: cardioramachandra@gmail.com
References
- Bıcakcıgil M, Aksu K, Kamali S, et al. Takayasu’s arteritis in Turkey – clinical and angiographic features of 248 patients. Clin Exp Rheumatol. 2009;27:S59-S564.
- Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994;120:919-929.
- Labarca C, Makol A, Crowson CS, Kermani TA, Matteson EL, Warrington KJ. Retrospective comparison of open versus endovascular procedures for Takayasu arteritis. J Rheumatol. 2016;43:427-432.
- Unnikrishnan M, Savlania A, Goura P, Tripathi RK. Aortic disease and management in India. In: Vascular Surgery. Springer International Publishing. 2017:65-71.
- Ovaert C, Gitenay E, Fraisse A. Challenges of treatment of aortic obstruction in Takayasu disease. Cardiol Young. 2015;25:803-805.
- Ng WF, Fantin F, Ng C, et al. Takayasu’s arteritis: a cause of prolonged arterial stiffness. Rheumatology (Oxford). 2006;45:741-745.
- Park MC, Lee SW, Park YB, Lee SK, Choi D, Shim WH. Post-interventional immunosuppressive treatment and vascular restenosis in Takayasu’s arteritis. Rheumatology. 2006;45:600-605.
- Tyagi S, Verma PK, Gambhir DS, Kaul UA, Saha R, Arora R. Early and long-term results of subclavian angioplasty in aortoarteritis (Takayasu disease): comparison with atherosclerosis. Cardiovasc Intervent Radiol. 1998;21:219-224.
- Tyagi S, Singh S, Mukhopadhyay S, Kaul UA. Self-and balloon-expandable stent implantation for severe native coarctation of aorta in adults. Am Heart J. 2003;146:920-928.
- Yagura M, Sano I, Akioka H, Hayashi M, Uchida H. Usefulness of percutaneous transluminal angioplasty for aortitis syndrome. Arch Intern Med. 1984;144:1465-1468.
- Sharma S, Pinto RJ. Fatal aortic rupture following balloon angioplasty of aortic restenosis in aortoarteritis. Cathet Cardiovasc Diagn. 1995;36:132-133.
- Deshmukh HL, Rathod KR, Sheth RJ, Garg A. Fatal aortic rupture complicating stent plasty in a case of aortoarteritis. Cardiovasc Intervent Radiol. 2003;26:496-498.
- Tyagi S, Rangasetty UC, Kaul UA. Endovascular treatment of aortic rupture during angioplasty for aortic in-stent restenosis in aortoarteritis. Catheter Cardiovasc Interv. 2003;58:103-106.
- Alcibar J, Blanco R, Fernandez L, et al. Elective implantation of covered stents for coarctation and recoarctation in adolescents and adults. Revista Española de Cardiología (Engl Ed). 2013;66:443-449.
- Hung JS, Chern MS, Wu JJ, et al. Short- and long-term results of catheter balloon percutaneous transvenous mitral commissurotomy. Am J Cardiol. 1991;67:854-862.
- Hung JS, Lau KW. Pitfalls and tips in Inoue-balloon mitral commissurotomy. Cathet Cardiovasc Diagn. 1996;37:188-199.
- Patil DV, Nabar AA, Sabnis GR, Phadke MS, Lanjewar CP, Kerkar PG. Percutaneous tricuspid valvotomy for pacemaker lead-induced tricuspid stenosis. Indian Heart J. 2015;67(Suppl 3):S115-S116. Epub 2016 Jan 14.
- Radhke W, Keane JF, Fellows KE, Lang P, Lock JE. Percutaneous balloon valvotomy of congenital pulmonary stenosis using oversized balloons. J Am Coll Cardiol. 1986;8:909-915.
- Kamioka N, Shirai S, Arita T. Impact of balloon sizing for clinical outcome in patients with severe aortic stenosis undergoing balloon aortic valvuloplasty. J Am Coll Cardiol. 2015;65.
- Rao PS. Neurologic complications following balloon angioplasty. Pediat Cardiol. 1993;14:63-64.
- Nichols AB, Smith R, Berke AD, Shlofmitz RA, Powers ER. Importance of balloon size in coronary angioplasty. J Am Coll Cardiol. 1989;13:1094-1100.
- Kim HJ, Do YS, Shin SW, et al. Percutaneous transluminal angioplasty of renal artery fibromuscular dysplasia: midterm results. Korean J Radiol. 2008;9:38-44.












