What Caused These Photodistributed Skin Changes?

A 65-year-old man presented for evaluation of skin changes on his neck and arms of 5 years’ duration. His past medical history was only significant for hypertension. He was initially treated with lisinopril and hydrochlorothiazide in 2005. Two years later his medications were changed to a combination drug containing triamterene and hydrochlorothiazide. Diltiazem was added in 2010, the dose ranged from 120 to 240 mg per day. The other medication was subsequently discontinued and his blood pressure was maintained with 240 mg of diltiazem daily.
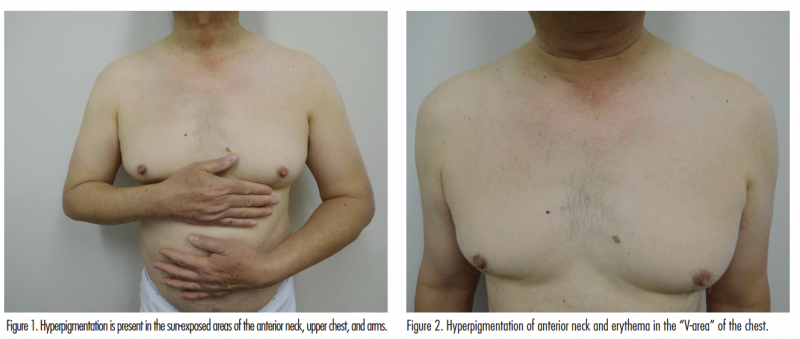
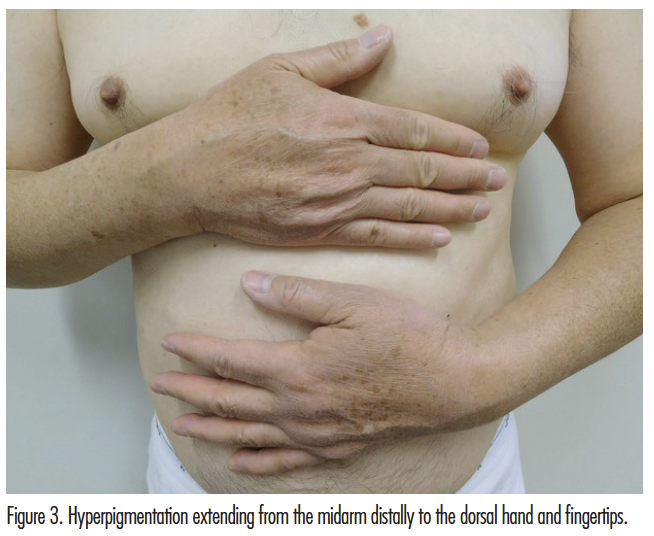
Cutaneous examination showed hyperpigmentation and erythema on the anterior neck and upper chest (Figures 1 and 2). There is brown hyperpigmentation of both upper extremities extending from the mid proximal arm to the dorsal hands and fingertips (Figures 1 and 3).
What is Your Diagnosis?
Find the answer on Page 2
Diagnosis: Diltiazem-Associated Photodistributed Hyperpigmentation
Diltiazem is commonly used in the management of cardiovascular diseases. It is a calcium channel blocker that decreases cardiac contractility and causes vasodilation. It has infrequently been associated with cutaneous adverse effects including hyperpigmentation in sun-exposed areas, pruritus, Stevens-Johnson syndrome, urticaria, and vasculitis.1
History
Diltiazem was approved by the FDA for the management of hypertension in 1982. Shortly thereafter, a 76-year-old woman with psoriasis who experienced severe generalized erythema to psoralen–UV-A therapy (PUVA) 2 weeks after initiating diltiazem was described by Young and colleagues in 1990.2 The patient had been treated with PUVA without complications prior to the initiation of diltiazem.
Subsequently, multiple additional examples of this cutaneous side effect have been described. In one case series, the patients were primarily female (12/16), black (10/16), and the most common areas affected were the face (16/16), the neck (11/16), the forearms (5/16), and the chest (3/16).3 The onset of hyperpigmentation after the initiation of diltiazem therapy ranged from 1.5 months to 12.5 years. Our patient’s presentation occurred 5 years after initially receiving the medication.
Clinical Presentation
Diffuse hyperpigmentation of the face, neck, upper chest, and upper extremities may occur in patients receiving diltiazem (Figures 1-3). The lesions usually appear as slate-gray and/or reticulated patches in a photodistributed area.4
Pathology
Pathologic changes in the skin from the hyperpigmented areas of patients receiving diltiazem show effaced rete ridges, and vacuolar alteration of the basal layer, with lymphocytic infiltrates, pigmentary incontinence, and melanophages in the dermis.3,5,6
Clinical Differential Diagnosis
Common oral photosensitizing drugs include amiodarone, antibiotics (especially in the tetracycline class), diuretics, nonsteroidal anti-inflammatory drugs (especially derivatives of propionic acid), psoralens, and phenothiazine.7 Drugs that can result in gray pigmentation include amiodarone, antineoplastic agents (including cisplatin and hydroxyurea), minocycline, phenytoin, and tricyclic antidepressants (such as desipramine and imipramine).8 However, the clinical presentation and corresponding medical history of these conditions usually allow for them to be differentiated from diltiazem-associated hyperpigmentation.
Pathogenesis
Common mechanisms of drug-induced hyperpigmentation include (1) drug-induced inflammation leading to melanin accumulation; (2) drug accumulation; (3) drug-induced lipofuscin production; and (4) iron deposition due to drug-induced vessel damage.8
The pathogenesis of this disease remains to be established. One hypothesis is that sun exposure induces a free radical complex with either diltiazem or a drug metabolite because diltiazem has an absorption range in the UV-B spectrum.5,9 However, ultrastructural analysis shows no drug or metabolite deposits within the dermal cells which suggests that another mechanism of action may also contribute to this condition.4
Treatment
It is necessary to recognize that diltiazem is the causative agent resulting in the cutaneous eruption. Switching to another drug class to control the patient’s hypertension should be considered. Also, measures to provide protection from sun exposure (such as sunscreen and clothing) should be recommended. Other topical treatments may include chemical peels,5 hydroquinone,4,5 and tacrolimus.3
Prognosis
The hyperpigmentation often significantly fades or resolves completely when diltiazem is discontinued.4,10 However, the time until complete resolution may range from 3 weeks3 to 1 year.5
Our Patient
Our patient elected to continue taking diltiazem. However, he started to wear photoprotective clothing and buttoned his shirt to the collar. Yet, at 6-month follow-up, his hyperpigmentation still persists.
Conclusion
Diltiazem is a calcium channel blocker commonly used to treat hypertension. Cutaneous adverse events are extremely rare given the widespread use of the drug. However, a photodistributed, reticulated, and/or slate gray hyperpigmentation associated with diltiazem use may occur. Diltiazem-associated hyperpigmentation is most frequently described in dark-skinned women; the onset of symptoms after initiation of diltiazem can range from months to decades. It is important for dermatologists to identify the connection between diltiazem and this characteristic hyperpigmentation. Once the diagnosis is established and the drug is discontinued, the prognosis is excellent for full or near-complete resolution of the hyperpigmentation.
 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Cohen is with the department of dermatology at the University of California, San Diego in La Jolla, CA.
Disclosure: The authors report no relevant financial relationships.
References
1. Seggev JS, Lagstein Z. Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol. 1996;97(3):852-855.
2. Young L, Shehade SA, Chalmers RJ. Cutaneous reactions to diltiazem. Clin Exp Dermatol. 1990;15(6):467-467.
3. Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol. 2010;37(9):807-811.
4. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001;137(2):179-182.
5. Saladi RN, Cohen SR, Phelps RG, Persaud AN, Rudikoff D. Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Arch Dermatol. 2006;142(2):206-210.
6. Berendzen SM, Carey JD, Smith EB. Diltiazem-associated photodistributed hyperpigmentation in an elderly Hispanic female. Int J Dermatol. 2006;45(12):1450-1452.
7. Cohen PR. What is the cause of the tender erythema? The Dermatologist. 2015;23(8):47-50.
8. Eichenfield DZ, Cohen PR. Amitriptyline-induced cutaneous hyperpigmentation: case report and review of psychotropic drug-associated mucocutaneous hyperpigmentation. Dermatol Online J. 2016;22(2):6.
9. Hanson M, Petronic-Rosic V. Reticulated phototoxic eruption in a patient on long-term diltiazem therapy. J Drugs Dermatol. 2008;7(8):792-793.
10. Ramírez A, Pérez-Pérez L, Fernández-Redondo V, Toribio J. Photoallergic dermatitis induced by diltiazem. Contact Dermatitis. 2007;56(2):118-119.

A 65-year-old man presented for evaluation of skin changes on his neck and arms of 5 years’ duration. His past medical history was only significant for hypertension. He was initially treated with lisinopril and hydrochlorothiazide in 2005. Two years later his medications were changed to a combination drug containing triamterene and hydrochlorothiazide. Diltiazem was added in 2010, the dose ranged from 120 to 240 mg per day. The other medication was subsequently discontinued and his blood pressure was maintained with 240 mg of diltiazem daily.
Cutaneous examination showed hyperpigmentation and erythema on the anterior neck and upper chest (Figures 1 and 2). There is brown hyperpigmentation of both upper extremities extending from the mid proximal arm to the dorsal hands and fingertips (Figures 1 and 3).
What's Your Diagnosis?
Diagnosis: Diltiazem-Associated Photodistributed Hyperpigmentation
Diltiazem is commonly used in the management of cardiovascular diseases. It is a calcium channel blocker that decreases cardiac contractility and causes vasodilation. It has infrequently been associated with cutaneous adverse effects including hyperpigmentation in sun-exposed areas, pruritus, Stevens-Johnson syndrome, urticaria, and vasculitis.1
History
Diltiazem was approved by the FDA for the management of hypertension in 1982. Shortly thereafter, a 76-year-old woman with psoriasis who experienced severe generalized erythema to psoralen–UV-A therapy (PUVA) 2 weeks after initiating diltiazem was described by Young and colleagues in 1990.2 The patient had been treated with PUVA without complications prior to the initiation of diltiazem.
Subsequently, multiple additional examples of this cutaneous side effect have been described. In one case series, the patients were primarily female (12/16), black (10/16), and the most common areas affected were the face (16/16), the neck (11/16), the forearms (5/16), and the chest (3/16).3 The onset of hyperpigmentation after the initiation of diltiazem therapy ranged from 1.5 months to 12.5 years. Our patient’s presentation occurred 5 years after initially receiving the medication.
Clinical Presentation
Diffuse hyperpigmentation of the face, neck, upper chest, and upper extremities may occur in patients receiving diltiazem (Figures 1-3). The lesions usually appear as slate-gray and/or reticulated patches in a photodistributed area.4


Pathology
Pathologic changes in the skin from the hyperpigmented areas of patients receiving diltiazem show effaced rete ridges, and vacuolar alteration of the basal layer, with lymphocytic infiltrates, pigmentary incontinence, and melanophages in the dermis.3,5,6
Clinical Differential Diagnosis
Common oral photosensitizing drugs include amiodarone, antibiotics (especially in the tetracycline class), diuretics, nonsteroidal anti-inflammatory drugs (especially derivatives of propionic acid), psoralens, and phenothiazine.7 Drugs that can result in gray pigmentation include amiodarone, antineoplastic agents (including cisplatin and hydroxyurea), minocycline, phenytoin, and tricyclic antidepressants (such as desipramine and imipramine).8 However, the clinical presentation and corresponding medical history of these conditions usually allow for them to be differentiated from diltiazem-associated hyperpigmentation.
Pathogenesis
Common mechanisms of drug-induced hyperpigmentation include (1) drug-induced inflammation leading to melanin accumulation; (2) drug accumulation; (3) drug-induced lipofuscin production; and (4) iron deposition due to drug-induced vessel damage.8
The pathogenesis of this disease remains to be established. One hypothesis is that sun exposure induces a free radical complex with either diltiazem or a drug metabolite because diltiazem has an absorption range in the UV-B spectrum.5,9 However, ultrastructural analysis shows no drug or metabolite deposits within the dermal cells which suggests that another mechanism of action may also contribute to this condition.4
Treatment
It is necessary to recognize that diltiazem is the causative agent resulting in the cutaneous eruption. Switching to another drug class to control the patient’s hypertension should be considered. Also, measures to provide protection from sun exposure (such as sunscreen and clothing) should be recommended. Other topical treatments may include chemical peels,5 hydroquinone,4,5 and tacrolimus.3
Prognosis
The hyperpigmentation often significantly fades or resolves completely when diltiazem is discontinued.4,10 However, the time until complete resolution may range from 3 weeks3 to 1 year.5
Our Patient
Our patient elected to continue taking diltiazem. However, he started to wear photoprotective clothing and buttoned his shirt to the collar. Yet, at 6-month follow-up, his hyperpigmentation still persists.
Conclusion
Diltiazem is a calcium channel blocker commonly used to treat hypertension. Cutaneous adverse events are extremely rare given the widespread use of the drug. However, a photodistributed, reticulated, and/or slate gray hyperpigmentation associated with diltiazem use may occur. Diltiazem-associated hyperpigmentation is most frequently described in dark-skinned women; the onset of symptoms after initiation of diltiazem can range from months to decades. It is important for dermatologists to identify the connection between diltiazem and this characteristic hyperpigmentation. Once the diagnosis is established and the drug is discontinued, the prognosis is excellent for full or near-complete resolution of the hyperpigmentation.
 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Cohen is with the department of dermatology at the University of California, San Diego in La Jolla, CA.
Disclosure: The authors report no relevant financial relationships.
References
1. Seggev JS, Lagstein Z. Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol. 1996;97(3):852-855.
2. Young L, Shehade SA, Chalmers RJ. Cutaneous reactions to diltiazem. Clin Exp Dermatol. 1990;15(6):467-467.
3. Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol. 2010;37(9):807-811.
4. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001;137(2):179-182.
5. Saladi RN, Cohen SR, Phelps RG, Persaud AN, Rudikoff D. Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Arch Dermatol. 2006;142(2):206-210.
6. Berendzen SM, Carey JD, Smith EB. Diltiazem-associated photodistributed hyperpigmentation in an elderly Hispanic female. Int J Dermatol. 2006;45(12):1450-1452.
7. Cohen PR. What is the cause of the tender erythema? The Dermatologist. 2015;23(8):47-50.
8. Eichenfield DZ, Cohen PR. Amitriptyline-induced cutaneous hyperpigmentation: case report and review of psychotropic drug-associated mucocutaneous hyperpigmentation. Dermatol Online J. 2016;22(2):6.
9. Hanson M, Petronic-Rosic V. Reticulated phototoxic eruption in a patient on long-term diltiazem therapy. J Drugs Dermatol. 2008;7(8):792-793.
10. Ramírez A, Pérez-Pérez L, Fernández-Redondo V, Toribio J. Photoallergic dermatitis induced by diltiazem. Contact Dermatitis. 2007;56(2):118-119.

A 65-year-old man presented for evaluation of skin changes on his neck and arms of 5 years’ duration. His past medical history was only significant for hypertension. He was initially treated with lisinopril and hydrochlorothiazide in 2005. Two years later his medications were changed to a combination drug containing triamterene and hydrochlorothiazide. Diltiazem was added in 2010, the dose ranged from 120 to 240 mg per day. The other medication was subsequently discontinued and his blood pressure was maintained with 240 mg of diltiazem daily.
Cutaneous examination showed hyperpigmentation and erythema on the anterior neck and upper chest (Figures 1 and 2). There is brown hyperpigmentation of both upper extremities extending from the mid proximal arm to the dorsal hands and fingertips (Figures 1 and 3).
What's Your Diagnosis?
,
What Caused These Photodistributed Skin Changes?

A 65-year-old man presented for evaluation of skin changes on his neck and arms of 5 years’ duration. His past medical history was only significant for hypertension. He was initially treated with lisinopril and hydrochlorothiazide in 2005. Two years later his medications were changed to a combination drug containing triamterene and hydrochlorothiazide. Diltiazem was added in 2010, the dose ranged from 120 to 240 mg per day. The other medication was subsequently discontinued and his blood pressure was maintained with 240 mg of diltiazem daily.
Cutaneous examination showed hyperpigmentation and erythema on the anterior neck and upper chest (Figures 1 and 2). There is brown hyperpigmentation of both upper extremities extending from the mid proximal arm to the dorsal hands and fingertips (Figures 1 and 3).
What is Your Diagnosis?
Find the answer on Page 2
Diagnosis: Diltiazem-Associated Photodistributed Hyperpigmentation
Diltiazem is commonly used in the management of cardiovascular diseases. It is a calcium channel blocker that decreases cardiac contractility and causes vasodilation. It has infrequently been associated with cutaneous adverse effects including hyperpigmentation in sun-exposed areas, pruritus, Stevens-Johnson syndrome, urticaria, and vasculitis.1
History
Diltiazem was approved by the FDA for the management of hypertension in 1982. Shortly thereafter, a 76-year-old woman with psoriasis who experienced severe generalized erythema to psoralen–UV-A therapy (PUVA) 2 weeks after initiating diltiazem was described by Young and colleagues in 1990.2 The patient had been treated with PUVA without complications prior to the initiation of diltiazem.
Subsequently, multiple additional examples of this cutaneous side effect have been described. In one case series, the patients were primarily female (12/16), black (10/16), and the most common areas affected were the face (16/16), the neck (11/16), the forearms (5/16), and the chest (3/16).3 The onset of hyperpigmentation after the initiation of diltiazem therapy ranged from 1.5 months to 12.5 years. Our patient’s presentation occurred 5 years after initially receiving the medication.
Clinical Presentation
Diffuse hyperpigmentation of the face, neck, upper chest, and upper extremities may occur in patients receiving diltiazem (Figures 1-3). The lesions usually appear as slate-gray and/or reticulated patches in a photodistributed area.4


Pathology
Pathologic changes in the skin from the hyperpigmented areas of patients receiving diltiazem show effaced rete ridges, and vacuolar alteration of the basal layer, with lymphocytic infiltrates, pigmentary incontinence, and melanophages in the dermis.3,5,6
Clinical Differential Diagnosis
Common oral photosensitizing drugs include amiodarone, antibiotics (especially in the tetracycline class), diuretics, nonsteroidal anti-inflammatory drugs (especially derivatives of propionic acid), psoralens, and phenothiazine.7 Drugs that can result in gray pigmentation include amiodarone, antineoplastic agents (including cisplatin and hydroxyurea), minocycline, phenytoin, and tricyclic antidepressants (such as desipramine and imipramine).8 However, the clinical presentation and corresponding medical history of these conditions usually allow for them to be differentiated from diltiazem-associated hyperpigmentation.
Pathogenesis
Common mechanisms of drug-induced hyperpigmentation include (1) drug-induced inflammation leading to melanin accumulation; (2) drug accumulation; (3) drug-induced lipofuscin production; and (4) iron deposition due to drug-induced vessel damage.8
The pathogenesis of this disease remains to be established. One hypothesis is that sun exposure induces a free radical complex with either diltiazem or a drug metabolite because diltiazem has an absorption range in the UV-B spectrum.5,9 However, ultrastructural analysis shows no drug or metabolite deposits within the dermal cells which suggests that another mechanism of action may also contribute to this condition.4
Treatment
It is necessary to recognize that diltiazem is the causative agent resulting in the cutaneous eruption. Switching to another drug class to control the patient’s hypertension should be considered. Also, measures to provide protection from sun exposure (such as sunscreen and clothing) should be recommended. Other topical treatments may include chemical peels,5 hydroquinone,4,5 and tacrolimus.3
Prognosis
The hyperpigmentation often significantly fades or resolves completely when diltiazem is discontinued.4,10 However, the time until complete resolution may range from 3 weeks3 to 1 year.5
Our Patient
Our patient elected to continue taking diltiazem. However, he started to wear photoprotective clothing and buttoned his shirt to the collar. Yet, at 6-month follow-up, his hyperpigmentation still persists.
Conclusion
Diltiazem is a calcium channel blocker commonly used to treat hypertension. Cutaneous adverse events are extremely rare given the widespread use of the drug. However, a photodistributed, reticulated, and/or slate gray hyperpigmentation associated with diltiazem use may occur. Diltiazem-associated hyperpigmentation is most frequently described in dark-skinned women; the onset of symptoms after initiation of diltiazem can range from months to decades. It is important for dermatologists to identify the connection between diltiazem and this characteristic hyperpigmentation. Once the diagnosis is established and the drug is discontinued, the prognosis is excellent for full or near-complete resolution of the hyperpigmentation.
 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Cohen is with the department of dermatology at the University of California, San Diego in La Jolla, CA.
Disclosure: The authors report no relevant financial relationships.
References
1. Seggev JS, Lagstein Z. Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol. 1996;97(3):852-855.
2. Young L, Shehade SA, Chalmers RJ. Cutaneous reactions to diltiazem. Clin Exp Dermatol. 1990;15(6):467-467.
3. Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol. 2010;37(9):807-811.
4. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001;137(2):179-182.
5. Saladi RN, Cohen SR, Phelps RG, Persaud AN, Rudikoff D. Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Arch Dermatol. 2006;142(2):206-210.
6. Berendzen SM, Carey JD, Smith EB. Diltiazem-associated photodistributed hyperpigmentation in an elderly Hispanic female. Int J Dermatol. 2006;45(12):1450-1452.
7. Cohen PR. What is the cause of the tender erythema? The Dermatologist. 2015;23(8):47-50.
8. Eichenfield DZ, Cohen PR. Amitriptyline-induced cutaneous hyperpigmentation: case report and review of psychotropic drug-associated mucocutaneous hyperpigmentation. Dermatol Online J. 2016;22(2):6.
9. Hanson M, Petronic-Rosic V. Reticulated phototoxic eruption in a patient on long-term diltiazem therapy. J Drugs Dermatol. 2008;7(8):792-793.
10. Ramírez A, Pérez-Pérez L, Fernández-Redondo V, Toribio J. Photoallergic dermatitis induced by diltiazem. Contact Dermatitis. 2007;56(2):118-119.

A 65-year-old man presented for evaluation of skin changes on his neck and arms of 5 years’ duration. His past medical history was only significant for hypertension. He was initially treated with lisinopril and hydrochlorothiazide in 2005. Two years later his medications were changed to a combination drug containing triamterene and hydrochlorothiazide. Diltiazem was added in 2010, the dose ranged from 120 to 240 mg per day. The other medication was subsequently discontinued and his blood pressure was maintained with 240 mg of diltiazem daily.
Cutaneous examination showed hyperpigmentation and erythema on the anterior neck and upper chest (Figures 1 and 2). There is brown hyperpigmentation of both upper extremities extending from the mid proximal arm to the dorsal hands and fingertips (Figures 1 and 3).
What's Your Diagnosis?
Diagnosis: Diltiazem-Associated Photodistributed Hyperpigmentation
Diltiazem is commonly used in the management of cardiovascular diseases. It is a calcium channel blocker that decreases cardiac contractility and causes vasodilation. It has infrequently been associated with cutaneous adverse effects including hyperpigmentation in sun-exposed areas, pruritus, Stevens-Johnson syndrome, urticaria, and vasculitis.1
History
Diltiazem was approved by the FDA for the management of hypertension in 1982. Shortly thereafter, a 76-year-old woman with psoriasis who experienced severe generalized erythema to psoralen–UV-A therapy (PUVA) 2 weeks after initiating diltiazem was described by Young and colleagues in 1990.2 The patient had been treated with PUVA without complications prior to the initiation of diltiazem.
Subsequently, multiple additional examples of this cutaneous side effect have been described. In one case series, the patients were primarily female (12/16), black (10/16), and the most common areas affected were the face (16/16), the neck (11/16), the forearms (5/16), and the chest (3/16).3 The onset of hyperpigmentation after the initiation of diltiazem therapy ranged from 1.5 months to 12.5 years. Our patient’s presentation occurred 5 years after initially receiving the medication.
Clinical Presentation
Diffuse hyperpigmentation of the face, neck, upper chest, and upper extremities may occur in patients receiving diltiazem (Figures 1-3). The lesions usually appear as slate-gray and/or reticulated patches in a photodistributed area.4


Pathology
Pathologic changes in the skin from the hyperpigmented areas of patients receiving diltiazem show effaced rete ridges, and vacuolar alteration of the basal layer, with lymphocytic infiltrates, pigmentary incontinence, and melanophages in the dermis.3,5,6
Clinical Differential Diagnosis
Common oral photosensitizing drugs include amiodarone, antibiotics (especially in the tetracycline class), diuretics, nonsteroidal anti-inflammatory drugs (especially derivatives of propionic acid), psoralens, and phenothiazine.7 Drugs that can result in gray pigmentation include amiodarone, antineoplastic agents (including cisplatin and hydroxyurea), minocycline, phenytoin, and tricyclic antidepressants (such as desipramine and imipramine).8 However, the clinical presentation and corresponding medical history of these conditions usually allow for them to be differentiated from diltiazem-associated hyperpigmentation.
Pathogenesis
Common mechanisms of drug-induced hyperpigmentation include (1) drug-induced inflammation leading to melanin accumulation; (2) drug accumulation; (3) drug-induced lipofuscin production; and (4) iron deposition due to drug-induced vessel damage.8
The pathogenesis of this disease remains to be established. One hypothesis is that sun exposure induces a free radical complex with either diltiazem or a drug metabolite because diltiazem has an absorption range in the UV-B spectrum.5,9 However, ultrastructural analysis shows no drug or metabolite deposits within the dermal cells which suggests that another mechanism of action may also contribute to this condition.4
Treatment
It is necessary to recognize that diltiazem is the causative agent resulting in the cutaneous eruption. Switching to another drug class to control the patient’s hypertension should be considered. Also, measures to provide protection from sun exposure (such as sunscreen and clothing) should be recommended. Other topical treatments may include chemical peels,5 hydroquinone,4,5 and tacrolimus.3
Prognosis
The hyperpigmentation often significantly fades or resolves completely when diltiazem is discontinued.4,10 However, the time until complete resolution may range from 3 weeks3 to 1 year.5
Our Patient
Our patient elected to continue taking diltiazem. However, he started to wear photoprotective clothing and buttoned his shirt to the collar. Yet, at 6-month follow-up, his hyperpigmentation still persists.
Conclusion
Diltiazem is a calcium channel blocker commonly used to treat hypertension. Cutaneous adverse events are extremely rare given the widespread use of the drug. However, a photodistributed, reticulated, and/or slate gray hyperpigmentation associated with diltiazem use may occur. Diltiazem-associated hyperpigmentation is most frequently described in dark-skinned women; the onset of symptoms after initiation of diltiazem can range from months to decades. It is important for dermatologists to identify the connection between diltiazem and this characteristic hyperpigmentation. Once the diagnosis is established and the drug is discontinued, the prognosis is excellent for full or near-complete resolution of the hyperpigmentation.
 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Cohen is with the department of dermatology at the University of California, San Diego in La Jolla, CA.
Disclosure: The authors report no relevant financial relationships.
References
1. Seggev JS, Lagstein Z. Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol. 1996;97(3):852-855.
2. Young L, Shehade SA, Chalmers RJ. Cutaneous reactions to diltiazem. Clin Exp Dermatol. 1990;15(6):467-467.
3. Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol. 2010;37(9):807-811.
4. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001;137(2):179-182.
5. Saladi RN, Cohen SR, Phelps RG, Persaud AN, Rudikoff D. Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Arch Dermatol. 2006;142(2):206-210.
6. Berendzen SM, Carey JD, Smith EB. Diltiazem-associated photodistributed hyperpigmentation in an elderly Hispanic female. Int J Dermatol. 2006;45(12):1450-1452.
7. Cohen PR. What is the cause of the tender erythema? The Dermatologist. 2015;23(8):47-50.
8. Eichenfield DZ, Cohen PR. Amitriptyline-induced cutaneous hyperpigmentation: case report and review of psychotropic drug-associated mucocutaneous hyperpigmentation. Dermatol Online J. 2016;22(2):6.
9. Hanson M, Petronic-Rosic V. Reticulated phototoxic eruption in a patient on long-term diltiazem therapy. J Drugs Dermatol. 2008;7(8):792-793.
10. Ramírez A, Pérez-Pérez L, Fernández-Redondo V, Toribio J. Photoallergic dermatitis induced by diltiazem. Contact Dermatitis. 2007;56(2):118-119.
Diagnosis: Diltiazem-Associated Photodistributed Hyperpigmentation
Diltiazem is commonly used in the management of cardiovascular diseases. It is a calcium channel blocker that decreases cardiac contractility and causes vasodilation. It has infrequently been associated with cutaneous adverse effects including hyperpigmentation in sun-exposed areas, pruritus, Stevens-Johnson syndrome, urticaria, and vasculitis.1
History
Diltiazem was approved by the FDA for the management of hypertension in 1982. Shortly thereafter, a 76-year-old woman with psoriasis who experienced severe generalized erythema to psoralen–UV-A therapy (PUVA) 2 weeks after initiating diltiazem was described by Young and colleagues in 1990.2 The patient had been treated with PUVA without complications prior to the initiation of diltiazem.
Subsequently, multiple additional examples of this cutaneous side effect have been described. In one case series, the patients were primarily female (12/16), black (10/16), and the most common areas affected were the face (16/16), the neck (11/16), the forearms (5/16), and the chest (3/16).3 The onset of hyperpigmentation after the initiation of diltiazem therapy ranged from 1.5 months to 12.5 years. Our patient’s presentation occurred 5 years after initially receiving the medication.
Clinical Presentation
Diffuse hyperpigmentation of the face, neck, upper chest, and upper extremities may occur in patients receiving diltiazem (Figures 1-3). The lesions usually appear as slate-gray and/or reticulated patches in a photodistributed area.4


Pathology
Pathologic changes in the skin from the hyperpigmented areas of patients receiving diltiazem show effaced rete ridges, and vacuolar alteration of the basal layer, with lymphocytic infiltrates, pigmentary incontinence, and melanophages in the dermis.3,5,6
Clinical Differential Diagnosis
Common oral photosensitizing drugs include amiodarone, antibiotics (especially in the tetracycline class), diuretics, nonsteroidal anti-inflammatory drugs (especially derivatives of propionic acid), psoralens, and phenothiazine.7 Drugs that can result in gray pigmentation include amiodarone, antineoplastic agents (including cisplatin and hydroxyurea), minocycline, phenytoin, and tricyclic antidepressants (such as desipramine and imipramine).8 However, the clinical presentation and corresponding medical history of these conditions usually allow for them to be differentiated from diltiazem-associated hyperpigmentation.
Pathogenesis
Common mechanisms of drug-induced hyperpigmentation include (1) drug-induced inflammation leading to melanin accumulation; (2) drug accumulation; (3) drug-induced lipofuscin production; and (4) iron deposition due to drug-induced vessel damage.8
The pathogenesis of this disease remains to be established. One hypothesis is that sun exposure induces a free radical complex with either diltiazem or a drug metabolite because diltiazem has an absorption range in the UV-B spectrum.5,9 However, ultrastructural analysis shows no drug or metabolite deposits within the dermal cells which suggests that another mechanism of action may also contribute to this condition.4
Treatment
It is necessary to recognize that diltiazem is the causative agent resulting in the cutaneous eruption. Switching to another drug class to control the patient’s hypertension should be considered. Also, measures to provide protection from sun exposure (such as sunscreen and clothing) should be recommended. Other topical treatments may include chemical peels,5 hydroquinone,4,5 and tacrolimus.3
Prognosis
The hyperpigmentation often significantly fades or resolves completely when diltiazem is discontinued.4,10 However, the time until complete resolution may range from 3 weeks3 to 1 year.5
Our Patient
Our patient elected to continue taking diltiazem. However, he started to wear photoprotective clothing and buttoned his shirt to the collar. Yet, at 6-month follow-up, his hyperpigmentation still persists.
Conclusion
Diltiazem is a calcium channel blocker commonly used to treat hypertension. Cutaneous adverse events are extremely rare given the widespread use of the drug. However, a photodistributed, reticulated, and/or slate gray hyperpigmentation associated with diltiazem use may occur. Diltiazem-associated hyperpigmentation is most frequently described in dark-skinned women; the onset of symptoms after initiation of diltiazem can range from months to decades. It is important for dermatologists to identify the connection between diltiazem and this characteristic hyperpigmentation. Once the diagnosis is established and the drug is discontinued, the prognosis is excellent for full or near-complete resolution of the hyperpigmentation.
 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Cohen is with the department of dermatology at the University of California, San Diego in La Jolla, CA.
Disclosure: The authors report no relevant financial relationships.
References
1. Seggev JS, Lagstein Z. Photosensitivity skin reactions to calcium channel blockers. J Allergy Clin Immunol. 1996;97(3):852-855.
2. Young L, Shehade SA, Chalmers RJ. Cutaneous reactions to diltiazem. Clin Exp Dermatol. 1990;15(6):467-467.
3. Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol. 2010;37(9):807-811.
4. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol. 2001;137(2):179-182.
5. Saladi RN, Cohen SR, Phelps RG, Persaud AN, Rudikoff D. Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Arch Dermatol. 2006;142(2):206-210.
6. Berendzen SM, Carey JD, Smith EB. Diltiazem-associated photodistributed hyperpigmentation in an elderly Hispanic female. Int J Dermatol. 2006;45(12):1450-1452.
7. Cohen PR. What is the cause of the tender erythema? The Dermatologist. 2015;23(8):47-50.
8. Eichenfield DZ, Cohen PR. Amitriptyline-induced cutaneous hyperpigmentation: case report and review of psychotropic drug-associated mucocutaneous hyperpigmentation. Dermatol Online J. 2016;22(2):6.
9. Hanson M, Petronic-Rosic V. Reticulated phototoxic eruption in a patient on long-term diltiazem therapy. J Drugs Dermatol. 2008;7(8):792-793.
10. Ramírez A, Pérez-Pérez L, Fernández-Redondo V, Toribio J. Photoallergic dermatitis induced by diltiazem. Contact Dermatitis. 2007;56(2):118-119.










 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.

 Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.
Dr Qin is with the department of medicine at the University of California, San Diego in La Jolla, CA.














