ADVERTISEMENT
Can Ultrasound Debridement Facilitate Biofilm Removal From Diabetic Foot Ulcers?
A patient with diabetes mellitus has a 15 to 25 percent chance of developing a diabetic foot ulcer during his or her lifetime.1 Once the patient with diabetes develops an ulcer, there is an even higher rate of ulcer recurrence at 50 to 70 percent over five years.1 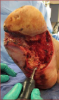 Diabetic foot ulcers are notoriously slow to heal and often lead to complications such as life-threatening infection and amputation.1 Traditionally, the mainstays of wound care include debridement, ensuring adequate vascular perfusion, eradicating infection and offloading diabetic foot ulcers.2 An often underestimated cause in the delay of wound healing is the development of biofilm. Researchers have estimated that 60 percent of chronic wounds are covered with biofilm.3 Biofilm occurs when bacteria colonize a wound and become encased in a protective coating of polysaccharides and lipids, termed glycocalyx, which is resistant to the patient’s immune system, systemic and topical antibiotics.1 Bacterial cells make up approximately 5 to 30 percent of biofilm and are typically polymicrobial.4 Biofilm thickness varies from a few micrometers to a few millimeters and bacteria encased in biofilm are approximately 1,000 times more resistant to antibiotics than in the planktonic form.4,5 Independent biofilm colonies form interconnected and sophisticated networks.6 Both Pseudomonas aeruginosa and Staphylococcus aureus, two common pathogens in diabetic foot ulcers, are known to form these structures.6 Methicillin-resistant Staphylcoccus aureus (MRSA) also lives in biofilm, further increasing the virulence of the bacteria.6 Biofilm can begin forming within hours and complex structures can form on a wound within two to four days.6 Biofilm clusters can break free and migrate to colonize new areas of the wound.4 Bacteria in biofilm communicate with each other through signaling molecules, termed quorum sensing.4 Pseudomonas aeruginosa uses quorum sensing for defense and can actually paralyze and lyse neutrophils.4 Wound healing is delayed in the presence of biofilm for a number of reasons. Biofilm causes chronic inflammation with the prolonged presence of neutrophils.4 The neutrophils prevent keratinocyte migration and release destructive reactive oxygen species and proteinases.4 Keratinocytes produce more matrix metalloproteinases in biofilm, which leads to destruction of the extracellular matrix and decreases growth factors.4 A microscope is often not necessary to detect biofilm as the presence of slough, shininess to an ulcer, malodor or necrotic tissue is likely an indication that biofilm is present.7 Superficial wound swab cultures often do not detect bacteria within biofilms, making swabs of little value.6 Deep tissue culture remains the most effective way of determining biofilm pathogens.6 Effective oral antibiotic treatment would require a much higher dose than what would be required if bacteria were not encased in biofilm, making antibiosis difficult.4 Topical antibiotics would also need to be polymicrobial and often do not penetrate the thick biofilm membrane.4
Diabetic foot ulcers are notoriously slow to heal and often lead to complications such as life-threatening infection and amputation.1 Traditionally, the mainstays of wound care include debridement, ensuring adequate vascular perfusion, eradicating infection and offloading diabetic foot ulcers.2 An often underestimated cause in the delay of wound healing is the development of biofilm. Researchers have estimated that 60 percent of chronic wounds are covered with biofilm.3 Biofilm occurs when bacteria colonize a wound and become encased in a protective coating of polysaccharides and lipids, termed glycocalyx, which is resistant to the patient’s immune system, systemic and topical antibiotics.1 Bacterial cells make up approximately 5 to 30 percent of biofilm and are typically polymicrobial.4 Biofilm thickness varies from a few micrometers to a few millimeters and bacteria encased in biofilm are approximately 1,000 times more resistant to antibiotics than in the planktonic form.4,5 Independent biofilm colonies form interconnected and sophisticated networks.6 Both Pseudomonas aeruginosa and Staphylococcus aureus, two common pathogens in diabetic foot ulcers, are known to form these structures.6 Methicillin-resistant Staphylcoccus aureus (MRSA) also lives in biofilm, further increasing the virulence of the bacteria.6 Biofilm can begin forming within hours and complex structures can form on a wound within two to four days.6 Biofilm clusters can break free and migrate to colonize new areas of the wound.4 Bacteria in biofilm communicate with each other through signaling molecules, termed quorum sensing.4 Pseudomonas aeruginosa uses quorum sensing for defense and can actually paralyze and lyse neutrophils.4 Wound healing is delayed in the presence of biofilm for a number of reasons. Biofilm causes chronic inflammation with the prolonged presence of neutrophils.4 The neutrophils prevent keratinocyte migration and release destructive reactive oxygen species and proteinases.4 Keratinocytes produce more matrix metalloproteinases in biofilm, which leads to destruction of the extracellular matrix and decreases growth factors.4 A microscope is often not necessary to detect biofilm as the presence of slough, shininess to an ulcer, malodor or necrotic tissue is likely an indication that biofilm is present.7 Superficial wound swab cultures often do not detect bacteria within biofilms, making swabs of little value.6 Deep tissue culture remains the most effective way of determining biofilm pathogens.6 Effective oral antibiotic treatment would require a much higher dose than what would be required if bacteria were not encased in biofilm, making antibiosis difficult.4 Topical antibiotics would also need to be polymicrobial and often do not penetrate the thick biofilm membrane.4
An Overview Of Surgical Debridement
Debridement is essential to wound bed preparation for removing necrotic tissue, bacteria and biofilm in wounds. Forms of debridement include autolytic (endogenous proteolysis), mechanical (wet to dry dressings), enzymatic (collagenases), biological (maggots) and surgical.9 Surgical debridement is often the most effective and efficient method.9 Surgical debridement also stimulates angiogenesis and eliminates matrix metalloproteinases, which exist in increased proportion in diabetic foot ulcers, causing destruction of healthy collagen.1 Often, physicians employ advanced therapies such as tissue-engineered skin equivalents to accelerate and promote wound healing.2 A healthy granular wound bed free of biofilm is essential or the costly skin substitute will ultimately fail. Negative pressure wound therapy has been effective for wound bed preparation in post-surgical diabetic wounds but not for chronic non-healing ulcers colonized with biofilm.2 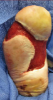 Surgical debridement has traditionally consisted of manual use of surgical tools such as a scalpel, curette or rongeur. One would usually irrigate the wound with copious amounts of saline after debridement. A commonly used product is the Pulsavac Plus Surgical Wound Debridement System (Zimmer), which provides adequate wound irrigation and removal of debris.10 For the past several years, Versajet (Smith and Nephew), a high-powered parallel water jet, has been in wide use.9 Researchers have shown the Versajet reduces biofilm in wounds and adequately prepares wound beds for skin grafts.11 The Versajet has the ability to increase debridement depth millimeter by millimeter and decrease debridement time by 39 percent.12
Surgical debridement has traditionally consisted of manual use of surgical tools such as a scalpel, curette or rongeur. One would usually irrigate the wound with copious amounts of saline after debridement. A commonly used product is the Pulsavac Plus Surgical Wound Debridement System (Zimmer), which provides adequate wound irrigation and removal of debris.10 For the past several years, Versajet (Smith and Nephew), a high-powered parallel water jet, has been in wide use.9 Researchers have shown the Versajet reduces biofilm in wounds and adequately prepares wound beds for skin grafts.11 The Versajet has the ability to increase debridement depth millimeter by millimeter and decrease debridement time by 39 percent.12
Exploring The Mechanisms Of Ultrasound Debridement
A further advance in wound care has been the development of contact ultrasound wound debridement systems. The term ultrasound derives from the frequency of the pressure waves the systems create when the operative tips contact tissue. These frequencies are generally above 20,000 cycles/second (Hz). Any frequency above those that the human ear can discern is considered an ultrasonic frequency. Since the upper frequency range for most people is 16 to 20,000 Hz, the resulting pressure waves are ultrasonic. In practice, the ultrasonic wound debrider systems fall into two categories, low frequency ultrasound and high frequency ultrasound. Low frequency systems operate between 20 and 100 KHz whereas high frequency systems operate in the range of 100 KHz up into the megahertz (MHz) range. Some ultrasonic wound treatment systems, such as the MIST Therapy system (Celleration), operate on a non-contact basis whereas ultrasound vibrations of a probe tip atomize a fluid such as saline and spray it onto the wound bed. The ultrasound energy contained in the vibrating droplet transfers into the tissue as the drop touches the surface. Others are contact type systems wherein a vibrating probe tip touches the wound bed directly. Low frequency contact type systems deliver higher energy densities to the wound bed, resulting in removal of biofilm and eschar as well as providing enhanced biocidal effects. Researchers have shown that ultrasound kills bacteria on wound surfaces.13 The two main mechanisms are cavitation and microstreaming.13 Cavitation is the creation of microbubbles in a fluid medium. With low frequency contact ultrasound, these bubbles emanate from the device’s operative vibrating tip touching the tissue itself. This creates some gaseous bubbles and then the bubbles collapse rapidly once they leave the proximity of the distal tip surface. Others are actually gas bubbles present in the tissue, which grow and shrink within the pulsating pressure field until they collapse on their own. Microbubbles affect bacteria by increasing temperature, inducing mechanical stress and/or free radical production.14 When either type of microbubbles collapse, they release pressure and energy very quickly. This energy release is bactericidal as it disrupts cell walls and otherwise renders the bacteria unviable.14 Microstreaming is the movement of fluid at rapid speeds at the intercellular level. Microstreaming causes gaseous bodies to vibrate forcefully enough to disrupt cell membranes.13,14 Low frequency ultrasound can clinically aid in the healing of chronic wounds. Cavitation and microstreaming promote collagen synthesis and improve strength, promote angiogenesis, and stimulate fibroblasts and macrophages.13 In a study of 17 patients, Breuing and colleagues found that in nine patients (53 percent), wounds healed primarily or after a skin graft, and six patients (35 percent) experienced at least a 50 percent wound reduction.7 Another study of 163 patients by Kavros and colleagues demonstrated that 53 percent of patients treated with the MIST Therapy System healed in comparison to 32 percent treated with standard wound care alone.15
A Closer Look At Emerging Ultrasonic Debridement Devices
Two relatively new low frequency contact ultrasound debridement systems are the SonicOne O.R. system (Misonix) and the Qoustic Wound Therapy System (Arobella Medical). In the in vitro study by Karau and colleagues, the authors found that the Qoustic Wound Therapy System effectively killed Pseudomonas aeruginosa, Staphylococcus epidermidis and Staphylococcus aureus, and decreased biofilm.13 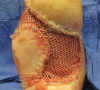 Both systems perform ultrasonic debridement via contact, cavitation and streaming although the Misonix Sonic One OR Surgical Debridement System also has enhanced micro-jackhammering effects as a function of its specialized tip designs.16,17 The Misonix Sonic One OR Surgical Debridement System generator converts voltage to an electrical signal, which transfers to the hand piece that contains piezoelectric crystals, and then converts the electrical signal to mechanical vibrations.16 The device amplifies these mechanical vibrations and acoustic energy transfers into the tissue by direct contact.16 From personal experience, the Misonix Sonic One ultrasound debridement system is efficient at removing biofilm and tissue necrosis in a controlled manner and promoting a healthy granular wound bed. After more trials and case studies demonstrate the positive outcome of the Misonix Sonic One Ultrasound Debridement System, we expect that it will become more widely used.
Both systems perform ultrasonic debridement via contact, cavitation and streaming although the Misonix Sonic One OR Surgical Debridement System also has enhanced micro-jackhammering effects as a function of its specialized tip designs.16,17 The Misonix Sonic One OR Surgical Debridement System generator converts voltage to an electrical signal, which transfers to the hand piece that contains piezoelectric crystals, and then converts the electrical signal to mechanical vibrations.16 The device amplifies these mechanical vibrations and acoustic energy transfers into the tissue by direct contact.16 From personal experience, the Misonix Sonic One ultrasound debridement system is efficient at removing biofilm and tissue necrosis in a controlled manner and promoting a healthy granular wound bed. After more trials and case studies demonstrate the positive outcome of the Misonix Sonic One Ultrasound Debridement System, we expect that it will become more widely used.
In Conclusion
Armstrong’s adage, “It’s not what you put on a wound but what you take off” can apply to ultrasound systems and the removal of biofilm as well.18 Many of the senior author’s patients with diabetic foot ulcers have done extremely well after debridement with the SonicOne O.R. system. Dr. Bowlby is a first-year resident at Yale University School of Medicine in New Haven, Ct. Dr. Blume is an Assistant Clinical Professor of Surgery in the Department of Surgery and an Assistant Clinical Professor of Orthopaedics and Rehabilitation in the Department of Orthopaedics, Section of Podiatric Surgery at the Yale University School of Medicine in New Haven, Ct. Dr. Blume is a Fellow of the American College of Foot and Ankle Surgeons. References 1. Alavi A, Sibbald RD, Mayer G, et al. Diabetic foot ulcers: part I. pathophysiology and prevention. J Am Acad Dermatol. 2014; 70(1):1.e1-18. 2. Alavi A, Sibbald RD, Mayer G, et al. Diabetic Foot Ulcers: Part II. Management. J Am Acad Dermatol. 2014; 70(1): 21.e1-24. 3. James G, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Rep Regen. 2008; 16(1):37–44. 4. Zhao GE, Usui ML, Lippman SI, et al. Biofilms and inflammation in chronic wounds. Adv Wound Care. 2013; 2(7):389-399. 5. Griswold J. Why diabetic wounds do not heal. Texas Heart Institute Journal. 2012; 39: 860-861. 6. Hall M, McGillicuddy E, Kaplan LJ. Biofilm: basic principles, pathophysiology, and implications for clinicians. Surgical Infections. 2014; 15(1):1-7. 7. Breuing K, Bayer L, Neuwalder J, Orgill DP. Early experience using low-frequency ultrasound in chronic wounds. Ann Plast Surg. 2005; 55(2):183–187. 8. Percival S, McCarty S, Hunt JA, Woods EG. A review of the scientific evidence for biofilms in wounds. Wound Rep Regen. 2012; 20(5):647–657. 9. Granick M, Posnett J, Jacoby M, et al. Efficacy and cost-effectiveness of a high-powered parallel waterjet for wound debridement. Wound Rep Regen. 2006; 14(4):394–397. 10. Zimmer Pulsavac Plus Wound Debridement Family Product Insert. Zimmer, 2008. 11. Bibbo C. Versajet hydrosurgery technique for the preparation of full thickness skin grafts and the creation of retrograde split thickness skin grafts. J Foot Ankle Surg. 2010; 49(4):404–407. 12. Kim P, Steinburg J. Wound care: biofilm and its impact on the latest treatment modalities for ulcerations of the diabetic foot. Semin Vasc Surg. 2012; 25(2):70-74. 13. Karau M, Piper KE, Steckleberg JM, et al. In vitro activity of the Qoustic Wound Therapy System against planktonic and biofilm bacteria. Adv Skin Wound Care. 2010; 23(7):316-320. 14. Scherba G, Weigel RM, O’Brien WD Jr., et al. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl Environ Microbiol. 1991; 57(7):2079-2084. 15. Kavros S, Liedl DE, Boon AJ, et al. Expedited wound healing with noncontact, low-frequency ultrasound therapy in chronic wounds: a retrospective analysis. Adv Skin Wound Care. 2008; 21(9):416-423. 16. Misonix Sonic One OR Surgical Debridement System Product Insert. Misonix, 2012. 17. Qoustic Wound Therapy System Product Insert. Arobella Medical, 2012. 18. Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It’s not what you put on, but what you take off: techniques for debriding and off-loading the diabetic foot wound. Clin Infect Dis. 2004; 39(Suppl2):S92–9. Additional Reference 19. Hess C, Howard MA, Attinger CE. A review of mechanical adjuncts in wound healing: hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Ann Plast Surg. 2003; 51(2):210-218.











