ADVERTISEMENT
Migraine Headaches Following Mitral Valvuloplasty: Koch’s Postulates Finally Satisfied?
Download a PDF of this article.
ABSTRACT: The relationship between patent foramen ovale and migraine headache is a topic surrounded by much controversy. We present the case of a 45-year-old woman who underwent successful percutaneous mitral balloon valvuloplasty for rheumatic mitral valve stenosis, but had an immediate postprocedural course complicated by refractory migraine headaches. Interestingly, resolution of her headaches coincided with the spontaneous closure of the interatrial communication created during valvuloplasty. This suggests interatrial flow as an important trigger for migraine headaches in this patient.
J INVASIVE CARDIOL 2013;25(6):E120-E123
Key words: PFO, migraine
_________________________________________
Migraine headache is a debilitating clinical disorder that significantly impacts quality of life, and exerts a tremendous economic cost in terms of work-days lost. Though the therapeutic options for migraines have grown over the last two decades, a large population of chronic migrane sufferers continues to utilize emergency services at a significant cost to society.1-4 Though the precise etiology of migraine headaches has not been elucidated, the association of patent foramen ovale (PFO) closure and the cessation of migraines was first described by Wilmshurst and colleagues as an incidental finding while examining the effect of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness.5 Several studies before and after this report have suggested an increased prevalence of PFO in patients with migraine headaches.6 Subsequent studies have further delineated that the largest association with PFO is in patients who have migraine with aura, as these patients appear twice as likely to have a PFO present when compared to a control group. Migraine patients without aura have PFO prevalence equal to controls.7,8
When percutaneous closure of PFO following stroke or transient ischemic attack first became routine, anecdotal reports were published describing patients who experienced migraine relief following closure. Retrospective studies utilizing questionnaires to assess the impact of closure on migraine supported a potential role of PFO in the pathogenesis of refractory migraine.9,10 Based on these promising findings, the MIST I randomized clinical trial of percutaneous closure of PFO using the BioSTAR device (NMT Medical, Inc) was performed. Study design was ambitious, utilizing sham closure in the non-closure group to avoid the potentially large placebo effect of closure. Shortcomings aside, including the presence of residual shunt in a substantial number of patients, the study failed to reach its primary endpoint. Though a trend toward fewer symptoms was seen with closure, resolution of migraine occurred with equal frequency in both study groups.11 On the heels of this publication, an epidemiological study from a well-studied cohort questioned whether an association between migraine and patent foramen ovale even existed.12 Of note, at least one large randomized trial continues to recruit patients to study the association utilizing a different device, and will hopefully shed greater light on the role of PFO closure.13
Determination of causality. Microbiologist Robert Koch defined a series of postulates in 1890 as a means of establishing a causal relationship between microbes and disease.14 Since this critical publication, Koch’s postulates have gained acceptance as a method of determining the etiology of various disease processes.15 Modifying these postulates to apply to PFO in the pathogenesis of migraine, the following steps would apply:
- The suspected characteristic (PFO) must be found more frequently in persons with the disease under investigation (migraine) than in persons without the disease.
- Elimination of the characteristic under investigation should lead to a measurable improvement in disease (closure of PFO should result in migraine improvement).
- Reintroduction of the entity should lead to recurrence of clinical disease (septostomy should be provocative in susceptible patients).
The first two postulates have arguably been satisfied for PFO and migraine headache. We describe a patient case that we believe could satisfy the remaining postulate.
Case Report. A 45-year-old woman with symptomatic rheumatic heart disease was referred to our hospital for consideration of percutaneous balloon mitral valvuloplasty. Preprocedural echocardiography showed a moderately thickened valve with moderately restricted leaflet motion, mild calcification, and mild subvalvular involvement (calculated Wilkin’s score, 8) with 1+ mitral regurgitation. The mean mitral valve gradient was 11 mm Hg with a peak gradient of 17 mm Hg and a calculated valve area of 1.1 cm2 by planimetry. There was mild left atrial enlargement, mild-to-moderate pulmonary hypertension (estimated right ventricular systolic pressure, 50 mm Hg) and normal biventricular size and function. A transesophageal echocardiogram further clarified the rheumatic involvement. There was no left atrial appendage thrombus present and saline microcavitation (bubble) study and color Doppler were negative for right-to-left shunt.
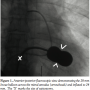 The patient underwent transthoracic echocardiographic and fluoroscopic-guided balloon valvuloplasty (Figure 1) using a standard transseptal technique and a 28 mm Inoue Balloon (Toray Industries). After valvuloplasty, the mean mitral gradient measured by simultaneous sampling in the left atrium and left ventricle was reduced from 9 mm Hg to 4 mm Hg, and the calculated valve area increased from 1.2 cm2 to 1.9 cm2. The mitral regurgitation by ventriculography remained unchanged (1+ before and after valvuloplasty). A shunt run at the end of the procedure showed a Qp/Qs of 1.2:1 (no shunt was present at the beginning of the procedure). The patient did well overnight following the procedure, and an echocardiogram the following morning showed a mean mitral valve gradient of 5 with a peak gradient of 9 and mild mitral regurgitation. Note was made at that time of “a small atrial septal defect” on both the apical 4-chamber and subcostal views with evidence of left-to-right
The patient underwent transthoracic echocardiographic and fluoroscopic-guided balloon valvuloplasty (Figure 1) using a standard transseptal technique and a 28 mm Inoue Balloon (Toray Industries). After valvuloplasty, the mean mitral gradient measured by simultaneous sampling in the left atrium and left ventricle was reduced from 9 mm Hg to 4 mm Hg, and the calculated valve area increased from 1.2 cm2 to 1.9 cm2. The mitral regurgitation by ventriculography remained unchanged (1+ before and after valvuloplasty). A shunt run at the end of the procedure showed a Qp/Qs of 1.2:1 (no shunt was present at the beginning of the procedure). The patient did well overnight following the procedure, and an echocardiogram the following morning showed a mean mitral valve gradient of 5 with a peak gradient of 9 and mild mitral regurgitation. Note was made at that time of “a small atrial septal defect” on both the apical 4-chamber and subcostal views with evidence of left-to-right 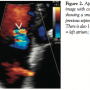 shunting by color Doppler (Figure 2). The patient was subsequently discharged home. During routine clinical follow-up 1 month later, a review of systems revealed the patient was now experiencing migraine headaches that began a few days after her discharge. She had been seen by a neurologist, who prescribed abortive therapy to use as needed. She reported having 3-4 headaches a week that generally began with an aura of flashing lights and odd scents, followed by unilateral eye and left temporal throbbing. Brain magnetic resonance imaging scan and magnetic resonance angiogram were obtained and showed no stroke or vascular abnormalities. On further questioning, the patient revealed she had migraines as a child that caused her to frequently miss school, but had eventually grown out of them. A repeat echocardiogram was performed during her visit and showed a persistence of atrial level shunting. Color Doppler this time suggested bidirectional shunting and a bubble study confirmed right-to-left shunting.
shunting by color Doppler (Figure 2). The patient was subsequently discharged home. During routine clinical follow-up 1 month later, a review of systems revealed the patient was now experiencing migraine headaches that began a few days after her discharge. She had been seen by a neurologist, who prescribed abortive therapy to use as needed. She reported having 3-4 headaches a week that generally began with an aura of flashing lights and odd scents, followed by unilateral eye and left temporal throbbing. Brain magnetic resonance imaging scan and magnetic resonance angiogram were obtained and showed no stroke or vascular abnormalities. On further questioning, the patient revealed she had migraines as a child that caused her to frequently miss school, but had eventually grown out of them. A repeat echocardiogram was performed during her visit and showed a persistence of atrial level shunting. Color Doppler this time suggested bidirectional shunting and a bubble study confirmed right-to-left shunting.
 The patient was started on a baby aspirin and asked to return in 5 months. At the time of her follow-up, she reported that her migraines had fully resolved. She further described a decrease in frequency over the second to third month following septostomy and then a rather sudden and complete resolution, “like someone turned off a switch.” A repeat echocardiogram showed no color Doppler flow across the septum (Figure 3) and was followed by a negative bubble study (Figure 4). The patient has consequently been followed yearly (now 6 years postintervention) with no migraine recurrence. Subsequent echocardiography studies have revealed limited restenosis of the mitral valve, and no evidence of shunting across the septum on color Doppler imaging.
The patient was started on a baby aspirin and asked to return in 5 months. At the time of her follow-up, she reported that her migraines had fully resolved. She further described a decrease in frequency over the second to third month following septostomy and then a rather sudden and complete resolution, “like someone turned off a switch.” A repeat echocardiogram showed no color Doppler flow across the septum (Figure 3) and was followed by a negative bubble study (Figure 4). The patient has consequently been followed yearly (now 6 years postintervention) with no migraine recurrence. Subsequent echocardiography studies have revealed limited restenosis of the mitral valve, and no evidence of shunting across the septum on color Doppler imaging.
 Discussion. For years, clinicians have debated whether a cause-and-effect relationship exists between PFO and migraine headache. The foramen ovale is an interatrial channel, defined inferiorly by the septum primum and superiorly by the septum secundum. Its anatomic configuration facilitates the physiologic right-to-left shunting of oxygen-rich placental blood during fetal life. During birth, the increased left atrial pressure leads to apposition of septum primum and secundum to close the foramen. When closure does not occur, a one-way valve-like structure remains, referred to as a PFO.16 PFO is an extremely common clinical entity, with studies generally reporting prevalence between 17% and 27%.17 Most of these patients will never experience stroke, migraine, or platypnea-orthodeoxia. Published expert opinion suggests there is a subset of patients with migraine in whom PFO is an integral part of the pathophysiology. How to identify these patients, however, remains uncertain.
Discussion. For years, clinicians have debated whether a cause-and-effect relationship exists between PFO and migraine headache. The foramen ovale is an interatrial channel, defined inferiorly by the septum primum and superiorly by the septum secundum. Its anatomic configuration facilitates the physiologic right-to-left shunting of oxygen-rich placental blood during fetal life. During birth, the increased left atrial pressure leads to apposition of septum primum and secundum to close the foramen. When closure does not occur, a one-way valve-like structure remains, referred to as a PFO.16 PFO is an extremely common clinical entity, with studies generally reporting prevalence between 17% and 27%.17 Most of these patients will never experience stroke, migraine, or platypnea-orthodeoxia. Published expert opinion suggests there is a subset of patients with migraine in whom PFO is an integral part of the pathophysiology. How to identify these patients, however, remains uncertain.
Migraine headaches are a complex disorder in which a variety of biochemical, neurophysiologic, and genetic factors play a role. It is feasible to postulate that a right-to-left shunt (via a septal communication) could facilitate the transfer of vasoactive chemicals including serotonin, kinins, and nitric oxide (normally inactivated by the lungs before reaching the systemic circulation) to the brain and trigger neurovascular spasm and thus migraine. Multiple studies have identified an association between PFO and migraine. One study found the prevalence of right-to-left shunting by transcranial Doppler to be 41% in patients with migraine with aura, compared to 16% in healthy controls.18
The relationship between PFO and migraine appears strongest among patients with migraine with aura, while several studies have found similar prevalence of right-to-left shunt between migraine without aura and healthy controls. However, it remains unclear if the relationship is causal or simply a coexistence of the two conditions.19 Various studies have suggested short-term reduction in migraines following PFO closure,10,20-22 including Wilmshurst’s study, where migraine frequency was reduced (or completely resolved) in 18 out of 21 patients.5 A recent study suggested long-term reduction of migraine intensity and frequency in over 60% of patients who had at least moderate shunts present on baseline testing.23 Despite the provocative nature of these findings, the only randomized trial designed to examine PFO closure failed to demonstrate a significant benefit.11 Further randomized trials are actively enrolling, utilizing different devices to further clarify the role of closure.
In this case report, relapse of migraines in a patient previously experiencing this condition coincided with the creation of an atrial septal communication and resolved following spontaneous closure of the communication. Our report mirrors a recent study that identified migraines in 22 of 2069 patients (1.1%) status-post iatrogenic creation of an atrial septal communication for transseptal catheter ablation of atrial fibrillation.24 It is interesting to note that 19 of the 22 patients (86%) had complete resolution of their migraine symptoms at 1- to 2-year follow-up, as in our patient. While it is impossible to firmly establish cause and effect in our case, the coincidence of these events suggests an association that may complete Koch’s postulates linking the pathogenetic factor (PFO) and the clinical disease (migraine headache).
References
- Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999;159(8):813-818.
- Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-657.
- Edmeads J, Mackell JA. The economic impact of migraine: an analysis of direct and indirect costs. Headache. 2002;42(6):501-509.
- Gerth WC, Carides GW, Dasbach EJ, et al. The multinational impact of migraine symptoms on healthcare utilization and work loss. Pharmacoeconomics. 2001;19(2):197-206.
- Wilmshurst PT, Nightingale S, Walsh KP, Morrison WL. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet. 2000;356(9242):1648-1651.
- Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA study. Stroke. 2002;33(3):706-711.
- Anzola GP, Magoni M, Guindani M, et al. Potential source of cerebral embolism in migraine with aura: a transcranial doppler study. Neurology. 1999;52(8):1622-1625.
- Domitrz I, Mieszkowski J, Kaminska A. Relationship between migraine and patent foramen ovale: a study of 121 patients with migraine. Headache. 2007;47(9):1311-1318.
- Post MC, Thijs V, Herroelen L, Budts WI. Closure of patent foramen ovale is associated with a decrease in prevalence of migraine. Neurology. 2004;62(8):1439-1440.
- Schwerzmann M, Wiher S, Nedeltchev K, et al. Percutaneous closure of patent foramen ovale reduces the frequency of migraine attacks. Neurology. 2004;62(8):1399-1401.
- Dowson A, Mullen MJ, Peatfield R, et al. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117(11):1397-1404.
- Rundek T, Elkind MS, Di Tullio MR, et al. Patent foramen ovale and migraine: a cross-sectional study from the Northern Manhattan Study (NOMAS). Circulation. 2008;118(14):1419-1424.
- Tepper SJ, Cleves C, Taylor FR. Patent foramen ovale and migraine: association, causation, and implications of clinical trials. Curr Pain Headache Rep. 2009;13(3):221-226.
- Koch R. Die Aetiologie der Tuberkulose. Mitt Kaiser Gesundh. 1884;2:1-88.
- Evans AS. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976;49(2):175-195.
- Braunwald E. Atrial septal defect. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. 4th edition. Philadelphia: WB Saunders Company: 1992:906-908.
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59(1):17-20.
- Del Sette M, Angeli S, Leandri M, et al. Migraine with aura and right-to-left shunt on transcranial Doppler: a case-control study. Cerebrovasc Dis. 1998;8(6):327-330.
- Schwerzmann M, Nedeltchev K, Lagger F, et al. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology. 2005;65(9):1415-1418.
- Reisman M, Christofferson RD, Jesurum J, et al. Migraine headache relief after transcatheter closure of patent foramen ovale. J Am Coll Cardiol. 2005;45(4):493-495.
- Azarbal B, Tobis J, Suh W, et al. Association of interatrial shunts and migraine headaches: impact of transcatheter closure. J Am Coll Cardiol. 2005;45(4):489-492.
- Giardini A, Donti A, Formigari R, et al. Transcatheter patent foramen ovale closure mitigates aura migraine headaches abolishing spontaneous right-to-left shunting. Am Heart J. 2006;151(4):922.e1-e5.
- Trabattoni D, Fabbiocchi F, Montorsi P, et al. Sustained long-term benefit of patent foramen ovale closure on migraine. Catheter Cardiovasc Interv. 2011;77(4):570-574.
- Noheria A, Roshan J, Kapa S, et al. Migraine headaches following catheter ablation for atrial fibrillation. J Interv Card Electrophysiol. 2011;30(3):227-232.
_____________________________________
From the Cleveland Clinic Foundation, Cleveland, Ohio. Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Krasuski is a consultant, grant holder, and receives honoraria from Actelion and is an unpaid Scientific Advisory Board member for Ventriprint. Manuscript submitted October 12, 2012, provisional acceptance given December 4, 2012, final version accepted January 4, 2013.
Address for correspondence: Richard A. Krasuski, MD, FACC, FAHA, Director of Adult Congenital Heart Disease Services, Cardiovascular Medicine/J2-4, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195. Email: krasusr@ccf.org











