ADVERTISEMENT
The Impact of a Chronic Total Coronary Occlusion on Outcomes of Patients With an Implantable Cardioverter Defibrillator: Insights From the EXPLORE Trial
Abstract: A chronic total occlusion (CTO) may increase the risk of appropriate implantable cardioverter-defibrillator (ICD) therapy. Therefore, we evaluated all patients who received an ICD during 5-year follow-up in the EXPLORE trial. Of 22 ICD patients, twelve were randomized to CTO percutaneous coronary intervention (PCI) and 10 to no revascularizaton of the CTO. Appropriate ICD therapy occurred in 1 patient in the CTO-PCI group. Compared with the 280 non-ICD patients in the EXPLORE trial, the 22 ICD patients had higher risk of adverse cardiac events (32% vs 10%; P<.01) and death (18% vs 6%; P=.02). These results suggest that ICD patients with CTO are at risk of poor outcomes; however, their benefit from ICD implantation is questionable.
Key words: chronic total occlusion, implantable cardioverter defibrillator, percutaneous coronary intervention
In patients with chronic total occlusion (CTO) of a coronary artery, the risk of appropriate implantable cardioverter defibrillator (ICD) therapy is increased when compared with non-CTO patients.1,2 Here, we describe the characteristics of ICD recipients and the occurrence of ICD therapy in the randomized EXPLORE (Evaluating Xience and left ventricular function in PCI on occLusiOns after STEMI) trial.3 The study protocol and outcomes of the EXPLORE trial have been published previously.3 Briefly, a total of 302 patients with ST-segment elevation myocardial infarction (STEMI) and concurrent CTO in a non-infarction related artery were randomized to percutaneous coronary intervention (PCI) of the CTO or no revascularization of the CTO for at least 4 months. No significant difference was found in the primary endpoint of left ventricular function at 4-month follow-up.
Methods
In the current substudy, we evaluated all patients receiving an ICD during follow-up until October 2017. Specific information about ICD implantation, follow-up, and ICD therapy was requested retrospectively with clinical research forms from the participating sites. The choice of ICD system and settings was at the operator’s discretion. Generally, the devices were programmed with monitor zone <150-160 beats/min, ventricular tachycardia zone with a cut-off around 150-160 beats/min, and ventricular fibrillation zone >220 beats/min. The study endpoints were: (1) occurrence of appropriate ICD therapy in ICD recipients in EXPLORE (the ICD group); and (2) all-cause mortality and major adverse cardiac event (MACE; defined as a composite of cardiac death, myocardial infarction, and coronary artery bypass grafting) in the patients with ICD vs the patients with no ICD. Appropriate ICD therapy was defined as delivery of antitachycardia pacing or electrical shocks for life-threatening ventricular arrhythmias. Any intervention by the ICD that did not meet this criterion was considered inappropriate.
Results
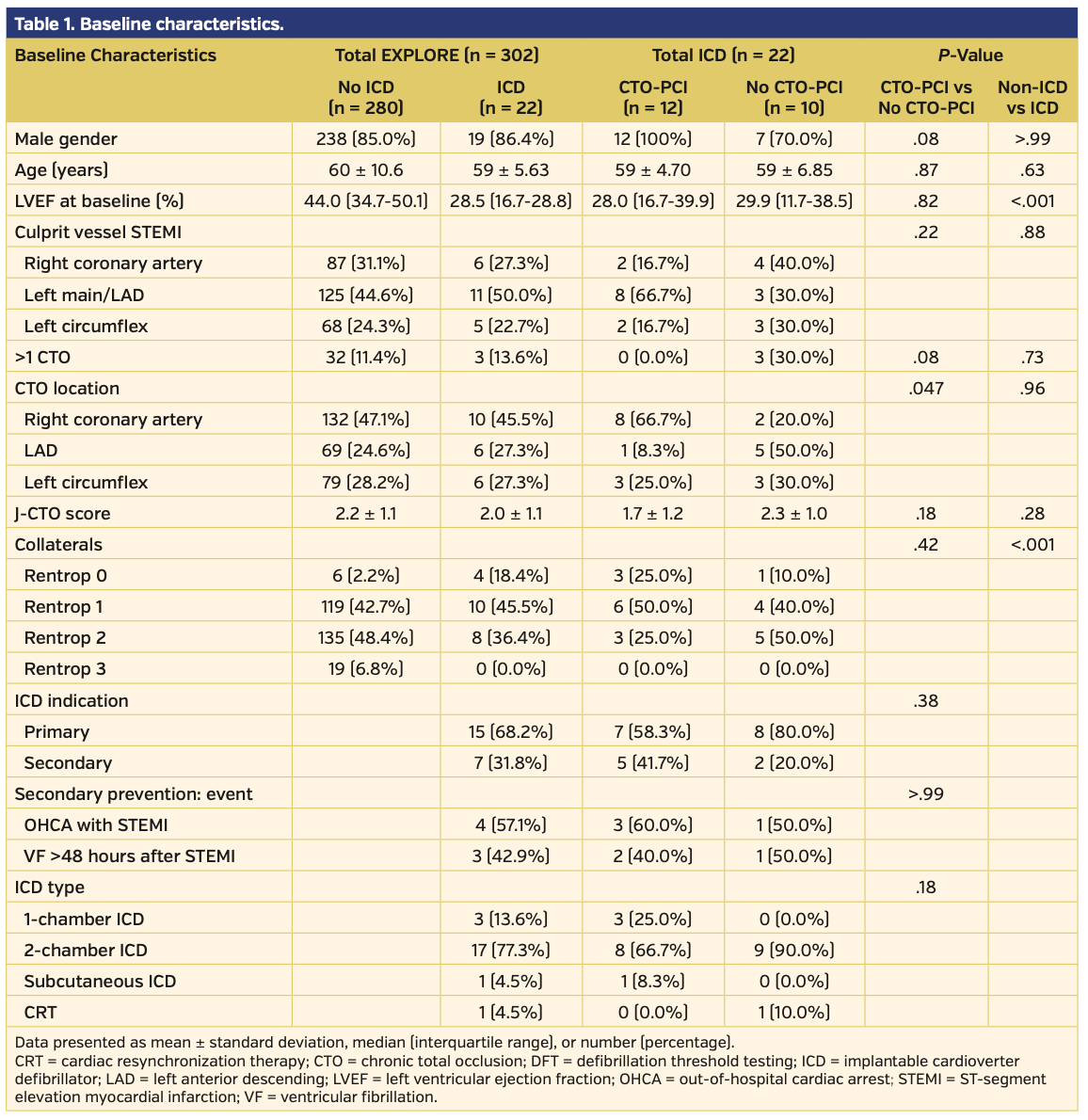
Of the 302 patients in the EXPLORE trial, a total of 22 (7%) underwent ICD implantation at a median of 167 days post randomization (interquartile range [IQR], 104-334 days). Twelve patients (55%) were randomized to the CTO-PCI group and 10 (45%) were randomized to the no-CTO PCI group. Baseline characteristics are displayed in Table 1. Median left ventricular ejection fraction was 28% (IQR, 17%-39%). During a median follow-up of 5 years (IQR, 4-6 years), nine patients experienced an arrhythmia within the monitor zone of the ICD, of which 6 patients had a supraventricular tachycardia and 5 patients had a non-sustained ventricular tachycardia. Appropriate ICD therapy occurred in 1 primary prevention patient in the CTO-PCI group. In total, during 4 episodes, this 2-chamber ICD delivered antitachycardia pacing 12 times for sustained ventricular tachycardia. No shocks were delivered. We calculated the MADIT-II risk score by assigning 1 point for each of the following criteria: age >70 years; New York Heart Association class >II; atrial fibrillation at baseline; QRS duration >120 ms; and creatinine >1.3 μmol/L (the latter as a substitute criterion for blood urea nitrogen since this is not commonly measured in the Netherlands).1,4 Thirteen patients (59%) had a MADIT-II risk score of 0 and 9 patients (41%) had a MADIT-II risk score of 1. No patient had a MADIT-II risk score >1.
The rate of ICD implantations per randomization group was comparable (8% in the CTO-PCI group vs 6% in the no-CTO-PCI group; P=.66). At baseline, ICD patients (n = 22) differed from no-ICD patients (n = 280) in left ventricular ejection fraction (28% [IQR, 22.2%] vs 44% [IQR, 16.2%], respectively; P<.001) (Table 1) and the absence of collaterals (18% vs 2%, respectively; P<.001). During 5-year follow-up, ICD patients were at a 3-fold higher risk of MACE (32% vs 10%; hazard ratio [HR], 2.72; 95% confidence interval [CI], 1.19-6.22; P=.02) and all-cause death (18% vs 6%; HR, 3.42; 95% CI, 1.26-9.28; P=.02) (Figure 1) than the no-ICD group.
Discussion
It is known that ICDs are most effective in patients with intermediate risk of death (MADIT-II score 1-2; HR, ± 0.40) and less effective in patients with very high risk of death (MADIT-II score ≥3; HR, 0.80) or very low risk of death (MADIT-II score 0; HR, 0.96) during the first 2 years post implantation.4 In our cohort, >50% had a MADIT-II risk score of 0. Thus, the predicted benefit of ICD implantation was low. Accordingly, ICD therapy barely occurred during follow-up. The incidence of life-threatening ventricular arrhythmias in our population during 5 years of follow-up was probably low, and almost no sudden cardiac deaths could have been prevented by the ICD. Conversely, the mortality in the ICD patients in EXPLORE was high, and presumably more frequently driven by terminal heart failure instead of sudden cardiac death.
Moreover, in our small cohort, revascularization of the CTO did not seem to influence the need for ICD implantation during follow-up, nor did the occurrence of ventricular arrhythmias in the monitor zone influence appropriate ICD therapy or death. To assess the effect of revascularization on ICD therapy, larger randomized studies are warranted.
Study limitations. Considerable limitations of our study should be acknowledged. First, there was low occurrence of ICD therapy in this small cohort. Previous studies reported ICD therapy rates ranging from 18% in primary prevention CTO patients to 50% in secondary prevention CTO patients.5,6 In our cohort of primary and secondary prevention patients, we observed an event rate of 4.5%. Hence, we could not assess the effect of revascularization of the CTO on ICD therapy. However, data on ICD therapy in a randomized cohort of CTO patients have not been described; thus, reporting these data is important for the clinical field. Second, since ICD implantation was not a primary endpoint in the main trial, there might be under-reporting of the number of ICD implantations.
Conclusion
In this post-STEMI CTO population randomized to CTO-PCI or no-CTO PCI who received an ICD during follow-up, high rates of MACE and mortality were observed compared to patients without ICD implantation. ICD implantation was not associated with an improvement of survival or MACE — even in these patients with low left ventricular ejection fractions. There was seemingly no effect from revascularization of the CTO on the need for ICD implantation. Future larger randomized studies are necessary to investigate the effect of revascularization on the occurrence of ICD therapy and the benefit of ICD implantation in these patients.
From the Amsterdam UMC, University of Amsterdam, Heart Center, Department of Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands.
Funding: The original EXPLORE trial was investigator initiated and funded by the Academic Medical Center and by a research grant from Abbott Vascular.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Henriques reports grant support from Abbott Vascular and AstraZeneca. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 3, 2019 and accepted September 17, 2019.
Address for correspondence: Prof Dr José P.S. Henriques, Amsterdam UMC, AMC Heart Center, Department of Cardiology, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. Email: j.p.henriques@amc.uva.nl
- Van Dongen IM, Yilmaz D, Elias J, et al. Evaluation of the impact of a chronic total coronary occlusion on ventricular arrhythmias and long-term mortality in patients with ischemic cardiomyopathy and an implantable cardioverter-defibrillator (the eCTOpy-in-ICD study). J Am Heart Assoc. 2018;7:e008609.
- Yap SC, Sakhi R, Theuns D, et al. Increased risk of ventricular arrhythmias in survivors of out-of-hospital cardiac arrest with chronic total coronary occlusion. Heart Rhythm. 2018;15:124-129.
- Henriques JP, Hoebers LP, Ramunddal T, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68:1622-1632.
- Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288-296.
- Nombela-Franco L, Iannaccone M, Anguera I, et al. Impact of chronic total coronary occlusion on recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter-defibrillator recipients (VACTO Secondary Study): insights from coronary angiogram and electrogram analysis. JACC Cardiovasc Interv. 2017;10:879-888.
- Nombela-Franco L, Mitroi CD, Fernandez-Lozano I, et al. Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention: impact of chronic total coronary occlusion (VACTO primary study). Circ Arrhythm Electrophysiol. 2012;5:147-154.