Total Medical and Pharmacy Costs for Patients With Metastatic Castration- Sensitive Prostate Cancer Initiating Oral Androgen Receptor Pathway Inhibitors in the United States
Abstract
This descriptive study aimed to evaluate the total cost of care and components of health care resource use and costs in androgen receptor pathway inhibitor (ARPI)-naïve Medicare-insured patients with metastatic castration-sensitive prostate cancer (mCSPC) who initiated their first ARPI. Clinical data from US community urology practices (Precision Point Specialty Analytics) were linked with administrative claims (Komodo Research Database; January 1, 2016, to September 30, 2022) to selected Medicare-insured patients with mCSPC who newly initiated apalutamide, enzalutamide, or abiraterone acetate. Patients with ≥ 12 months of continuous insurance eligibility were followed from the date of first use of apalutamide, enzalutamide, or abiraterone acetate (index date) until the earliest of castration-resistance progression, treatment discontinuation, initiation of a new therapy, end of continuous insurance eligibility, or end of data availability. All-cause and prostate cancer (PC)–related health care costs per patient per year (PPPY; 2022 US dollars) were summarized using descriptive statistics. A total of 152 patients receiving apalutamide (mean on treatment duration of 7.3 months), 189 patients receiving enzalutamide (mean on treatment duration of 7.7 months), and 387 patients receiving abiraterone acetate (mean on treatment duration of 9.4 months) were included. Mean all-cause total costs PPPY were $128 187 (apalutamide), $145 382 (enzalutamide), and $114 959 (abiraterone acetate). Mean PC-related total costs PPPY were $116 416 (apalutamide), $132 236 (enzalutamide), and $100 338 (abiraterone acetate). Mean all-cause medical costs PPPY were $22 902 in the apalutamide cohort, $30 933 in the enzalutamide cohort, and $35 625 in the abiraterone acetate cohort. Similar trends were observed for mean PC-related medical costs PPPY: $15 067 (apalutamide), $20 968 (enzalutamide), and $24 760 (abiraterone acetate). Mean all-cause pharmacy costs PPPY were $105 285 (apalutamide), $114 450 (enzalutamide), and $79 334 (abiraterone acetate). Observed variations in medical costs between ARPIs may provide an early indicator of their ability to manage PC.
Introduction
Prostate cancer (PC) is the second leading cause of cancer death and the most common cancer type among men in the US, with an estimated 32 350 deaths and 299 010 new cases expected in 2024.1 Metastatic castration-sensitive PC (mCSPC) accounts for approximately 4% of prostate tumors.2 Although 5-year survival rates for localized PC remain near 100%, survival among patients with distant disease, including mCSPC, decreases to approximately 37%.3
Androgen deprivation therapy (ADT) via surgical or medical castration has long been the standard of care for mCSPC.4,5 Initial prostate-specific antigen (PSA)-based response rates to ADT exceed 80%,5,6 however, nearly all patients with mCSPC eventually progress to metastatic castration-resistant PC (mCRPC) within 2 to 3 years.7,8 More recently, androgen receptor pathway inhibitors (ARPIs), used in combination with ADT, have emerged as important additions to the therapeutic landscape for mCSPC.9 The ARPIs approved by the US Food and Drug Administration (FDA) include apalutamide (approved on September 17, 2019, for mCSPC),10-12 enzalutamide (approved on December 16, 2019, for mCSPC),13-15 abiraterone acetate plus prednisone (approved on February 7, 2018, for high-risk mCSPC),16,17 and most recently darolutamide plus docetaxel (approved on August 5, 2022, for mCSPC).18,19 Together with ADT, ARPIs improve survival outcomes and help to slow the progression of mCSPC to mCRPC.9,20
Although real-world evidence suggests apalutamide may offer superior clinical outcomes in patients with mCSPC compared with other ARPIs,21,22 recent health care resource use (HRU) and cost data, which provide important information for assessing these agents’ utility in mCSPC, are lacking. Previous studies have established the high economic burden of mCSPC23 and increased HRU and costs associated with progression from mCSPC to mCRPC,24,25 but have not evaluated the costs associated with different ARPIs.
The economic burden of mCSPC may be reduced by considering advanced treatment options and slowing progression from mCSPC to mCRPC. In addition, evaluating annual total cost of care and the relationship of medical costs to nonmedical costs may be one of the means of assessing the therapeutic value of treatment alternatives in the real-world setting, with suboptimal adherence to the prescribed regimen and increasing HRU or higher medical costs serving as indicators of lower therapeutic value for specific pharmaceutical agents.26,27
With the approval of these newer therapeutic agents for the treatment of mCSPC, it is important to study their impact on health care costs, particularly in a Medicare-insured population, to prepare for future health care needs of older adults in the US. Thus, this study aimed to evaluate the total cost of care and components of HRU and costs in ARPI-naïve Medicare-insured patients with mCSPC initiating their first ARPI.
Methods
Data Sources
Clinical data from more than 90 US private community-based urology practices (Precision Point Specialty [PPS] Analytics) were linked with administrative claims (Komodo Research Database [KRD]; January 1, 2016, to September 30, 2022) to select Medicare-insured patients with mCSPC who newly initiated apalutamide, enzalutamide, or abiraterone acetate. Practices included in PPS, which are distributed nationally across the US, represent 4000 active community urology providers, who have treated approximately 25 000 patients with mCSPC. The database includes patient demographics and clinical variables such as laboratory testing results, PC-related medical procedures, and dispensation information for ARPIs.
The KRD is a deidentified database sourced from a variety of payers and health care organizations in the US. The Komodo database contains over 320 million US patients across Medicaid, commercial, and Medicare insurers, and consists of information on insurance coverage, diagnosis and procedures received in inpatient and outpatient settings, along with prescription fills, and mortality data. Only closed claims data were used in HRU and cost analyses. Patients in PPS and KRD were linked by Datavant, which employed their patent-pending deidentification technology using machine learning validation to substitute private patient data with an encrypted token that cannot be reverse- engineered to reveal any personal information. This study was considered exempt research under 45 CFR § 46.104(d)(4) as it involved only the secondary use of data that were deidentified in compliance with the Health Insurance Portability and Accountability Act (HIPAA), specifically, 45 CFR § 164.514.
Study Design
A retrospective longitudinal cohort design was used to evaluate HRU and costs. The study design scheme is depicted in Figure 1. The index date was defined as the date of the first dispensation (from PPS) or paid pharmacy claim (from KRD) for apalutamide, enzalutamide, or abiraterone acetate. The start of the index treatment identification period was based on the FDA approval for each treatment for mCSPC (ie, apalutamide: September 17, 2019; enzalutamide: December 16, 2019; abiraterone acetate: February 7, 2018, for high-risk mCSPC). The baseline period was defined as the 12-month period preceding the index date. The follow-up period spanned from the index date until the earliest of progression to castration resistance (ie, mCRPC), discontinuation of the index ARPI (ie, using a 90-day gap in days of supply), initiation of a different ARPI or a radiopharmaceutical agent, end of continuous insurance eligibility, or end of data availability (September 30, 2022). No minimum follow-up time was imposed to reduce the potential impact of survivor bias. Concurrent ADT use was not required for inclusion in this study. Concurrent prednisone use was not required for inclusion in the abiraterone acetate cohort.

Study Population
Patients with mCSPC who newly initiated an ARPI (ie, apalutamide, enzalutamide, or abiraterone acetate) with Medicare insurance were of interest in this study. The combination of darolutamide and ADT was not included in the analyses because it had not been approved for use in the US during the time frame of this study. Patients were required to meet the following criteria: (1) ≥ 1 paid pharmacy claim (from KRD) or dispensation (from PPS) for an ARPI (ie, apalutamide, enzalutamide, or abiraterone acetate) on or after their respective FDA approvals; (2) no prescription for another ARPI observed before the index date; (3) had mCSPC on the index date; (4) ≥ 18 years of age on the index date; (5) ≥ 12 months of continuous closed insurance enrollment prior to the index date; and (6) Medicare insurance coverage on the index date. The exclusion criterion was no use of radiopharmaceutical therapy before or on the index date.
Patients were considered to have mCSPC if they had evidence of metastases observed any time before or on the index date, in the absence of castration resistance observed any time before or on the index date. Metastatic disease was defined based on bone, nodal, or visceral metastasis identified through derived variables from PPS or diagnosis codes from PPS and KRD. Castration resistance was assessed using a previously published algorithm.28
Study Measures
Patient demographics and clinical characteristics (ie, Quan- Charlson Comorbidity Index [Quan-CCI], comorbidities) were evaluated during the baseline period (ie, 12 months pre-index) using data from both PPS and KRD, as applicable.
All-cause and PC-related HRU and cost outcomes were evaluated separately during the baseline and follow-up periods based on medical and pharmacy claims from KRD. HRU categories included the number of inpatient admissions, number of inpatient days, number of days with emergency department visits, number of days with outpatient visits, number of days with pharmacy claims, and number of days with other services. PC-related HRU and costs were identified based on medical claims with diagnosis code for PC (International Classification of Diseases, 10th Revision, Clinical Modification: C61) or claims with a procedure code for ADT or other therapies for metastatic PC (ie, ARPIs, chemotherapies, estrogens, immunotherapies, poly ADP-ribose polymerase inhibitors, or radiopharmaceuticals).
All-cause and PC-related health care costs, including medical costs (ie, sum of inpatient, emergency department, outpatient, other costs), pharmacy costs, and total health care costs (sum of medical and pharmacy costs) were reported. All HRU and cost outcomes were reported per patient per year (PPPY), and costs were reported in 2022 US dollars and adjusted for inflation using the medical care component of the US Consumer Price Index. Costs were reported from the payer’s perspective.
Statistical Analyses
All-cause and PC-related HRU and health care costs were summarized using descriptive statistics. For categorical variables (eg, proportion of patients with an outpatient visit), frequencies and proportions were reported. The means, medians, and SD were reported for count variables (eg, number of outpatient visits) and continuous variables (eg, pharmacy costs). HRU and costs were reported separately for each index ARPI (ie, apalutamide, enzalutamide, and abiraterone acetate). This study was descriptive, and no comparisons of HRU and cost outcomes were made between index ARPIs (eg, no P values were generated).
Results
Demographic and Clinical Characteristics
The study included a total of 152 patients who initiated apalutamide, 189 patients who initiated enzalutamide, and 387 patients who initiated abiraterone acetate (Figure 2). The following values were similar across cohorts: mean age (apalutamide: 76.5 years; enzalutamide: 76.2 years; abiraterone acetate: 76.5 years); time between initial diagnosis and index date (apalutamide: 39.7 months; enzalutamide: 43.4 months; abiraterone acetate: 39.7 months); and Quan-CCI score (apalutamide: 9.1; enzalutamide: 9.6; abiraterone acetate: 9.8) (Table 1). Most patients were White (apalutamide: 64.5%; enzalutamide: 63.0%; abiraterone acetate: 71.6%) and had evidence of bone metastasis (apalutamide: 73.0%; enzalutamide: 76.7%; abiraterone acetate: 79.8%). Almost all patients (95.3%) who initiated abiraterone acetate had concurrent prednisone use. Patients were followed while on treatment for a mean of 7.3 months in the apalutamide cohort, 7.7 months in the enzalutamide cohort, and 9.4 months in the abiraterone acetate cohort.



Health Care Resource Utilization
During the baseline period, 12.5% of the apalutamide cohort, 20.6% of the enzalutamide cohort, and 18.9% of the abiraterone acetate cohort had an all-cause inpatient admission (Table 2). The mean number of all-cause admissions and days spent in an inpatient setting PPPY were 0.16 admissions and 1.01 days for the apalutamide cohort, 0.35 admissions and 2.92 days for the enzalutamide cohort, and 0.28 admissions and 1.65 days for the abiraterone acetate cohort. During the same time period, all-cause emergency department visits occurred in 43.4% of the apalutamide cohort (mean 1.09 visits PPPY), 54.0% of the enzalutamide cohort (1.32 visits PPPY), and 54.3% of the abiraterone acetate cohort (1.27 visits PPPY), and all-cause outpatient visits occurred in 100% of the apalutamide cohort (mean 25.18 visits PPPY), 99.5% of the enzalutamide cohort (33.46 visits PPPY), and 99.5% of the abiraterone acetate cohort (36.34 visits PPPY).

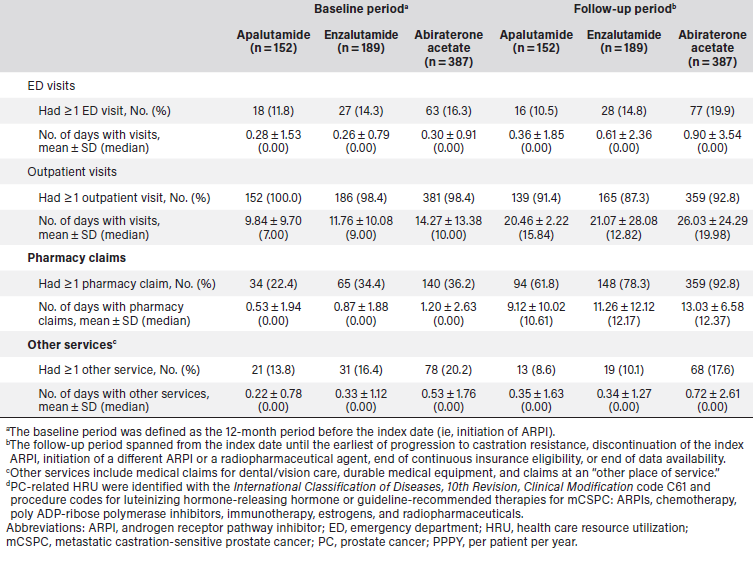
During the follow-up period, 10.5% of the apalutamide cohort, 9.0% of the enzalutamide cohort, and 15.2% of the abiraterone acetate cohort had an all-cause inpatient admission (Table 2). The mean number of all-cause admissions and days spent in an inpatient setting (PPPY) were 0.36 admissions and 2.14 days for the apalutamide cohort, 0.36 admissions and 3.67 days for the enzalutamide cohort, and 0.65 admissions and 4.30 days for the abiraterone acetate cohort. Similarly, PC-related inpatient admissions occurred in 7.9% of the apalutamide cohort (mean 0.28 admissions and 1.71 days PPPY), 6.3% of the enzalutamide cohort (mean 0.30 admissions and 2.01 days PPPY), and 13.2% of the abiraterone acetate cohort (mean 0.55 admissions and 3.95 days PPPY). All-cause emergency department visits occurred in 24.3% of the apalutamide cohort (mean 1.89 visits PPPY), 32.3% of the enzalutamide cohort (1.68 visits PPPY), and 36.4% of the abiraterone acetate cohort (1.95 visits PPPY) and all-cause outpatient visits occurred in 94.1% of the apalutamide cohort (mean 32.31 visits PPPY), 93.7% of the enzalutamide cohort (38.34 visits PPPY), and 95.6% of the abiraterone acetate cohort (43.77 visits PPPY). PC-related emergency department visits occurred in 10.5% of the apalutamide cohort (mean 0.36 visits PPPY), 14.8% of the enzalutamide cohort (0.61 visits PPPY), and 19.9% of the abiraterone acetate cohort (0.90 visits PPPY) and PC-related outpatient visits occurred in 91.4% of the apalutamide cohort (mean 20.46 visits PPPY), 87.3% of the enzalutamide cohort (21.07 visits PPPY), and 92.8% of the abiraterone acetate cohort (26.03 visits PPPY).
Health care costs
During the baseline period, the mean all-cause total costs PPPY were $18 216 for the apalutamide cohort, $23 633 for the enzalutamide cohort, and $27 595 for the abiraterone acetate cohort (Figure 3). Medical costs, which accounted for most of the baseline all-cause total costs, were $15 530 PPPY for the apalutamide cohort (85.3% of total costs), $21 113 PPPY for the enzalutamide cohort (89.3% of total costs), and $24 009 PPPY for the abiraterone acetate cohort (87.0% of total costs).

During the follow-up period, mean all-cause total costs PPPY were $128 187 for the apalutamide cohort, $145 382 for the enzalutamide cohort, and $114 959 for the abiraterone acetate cohort (Figure 3). Medical costs, which accounted for a smaller proportion of the follow-up all-cause total costs compared with the baseline period, were $22 902 PPPY for the apalutamide cohort, $30 933 PPPY for the enzalutamide cohort, and $35 625 PPPY for the abiraterone acetate cohort. All-cause medical costs were largely driven by outpatient costs, which were $16 696 PPPY for the apalutamide cohort (72.9% of medical costs), $20 148 PPPY for the enzalutamide cohort (65.1% of medical costs), and $24 559 PPPY for the abiraterone acetate cohort (69.0% of medical costs). All-cause inpatient and emergency department costs combined were $5922 PPPY for the apalutamide cohort, $10 388 PPPY for the enzalutamide cohort, and $10 832 PPPY for the abiraterone acetate cohort. Mean all-cause pharmacy costs PPPY were $105 285 for the apalutamide cohort, $114 450 for the enzalutamide cohort, and $79 334 for the abiraterone acetate cohort. Mean PC-related total costs PPPY were $116 416 for the apalutamide cohort, $132 236 for the enzalutamide cohort, and $100 338 for the abiraterone acetate cohort (Figure 4). Similarly, PC-related medical costs PPPY were $15 067 for the apalutamide cohort, $20 968 for the enzalutamide cohort, and $24 760 for the abiraterone acetate cohort.

Discussion
In this real-world descriptive analysis of Medicare-insured patients with mCSPC treated with an ARPI, annual all-cause and PC-related medical costs were similar between those treated with enzalutamide and abiraterone acetate, and numerically lower among those treated with apalutamide. Given similarities in baseline PC-related medical costs before initiating an ARPI, descriptively higher medical costs for patients after initiation of a cancer medication may indicate suboptimal medical management due to adverse events or insufficient treatment response, reflected in variations in HRU, including hospitalizations and emergency department visits.
This study adds to the small but growing body of literature demonstrating the high economic burden associated with mCSPC, particularly in the setting of progression from localized to metastatic disease.29,30 In a large retrospective cohort study using administrative claims data from patients insured through commercial, Medicare Advantage, and Medicare fee-for-service health plans, Ryan and colleagues found that mean all-cause health plan-paid costs were four to five times higher after the onset of metastases in patients with castration-sensitive PC.29 In a separate study using commercial insurance and Medicare claims data, disease progression from localized to mCSPC resulted in between two- and four-fold increases in total direct all-cause health care costs over an approximately 15-month mean follow-up period.30 Importantly, HRU and costs are shown to further increase when mCSPC progresses to mCRPC.24,31-33 In a longitudinal cohort study of patients who progressed to mCRPC, Kaye and colleagues found substantial increases in all-cause mean (SD) costs per patient per month (PPPM) from $4166 ($7548) to $8278 ($18 510), primarily driven by PC-related costs, such as pharmacy, outpatient, and imaging costs.25 Among Medicare-insured patients in the study, mean (SD) all-cause and PC-related costs PPPM increased from $3457 ($5412) to $6672 ($18 062) and $2222 ($3937) to $5455 ($16 163), respectively, following progression to mCRPC. These results suggest that effective interventions for patients with mCSPC that delay progression may help to alleviate the economic burden associated with mCRPC.
Medical costs, when used alongside clinical evidence, may help to provide a more comprehensive understanding of a medication’s therapeutic value by reflecting both its real-world economic impact and its effectiveness in clinical practice.26 In this study, medical costs were numerically lower for patients treated with apalutamide relative to those treated with enzalutamide and abiraterone acetate, which may suggest sufficient treatment response and reduced HRU during treatment in patients treated with apalutamide. This finding further supports the clinical benefit of apalutamide, which has been established in previous comparative real-world studies that used clinical data from community urology practices in the US. In one study, patients treated with apalutamide were 53% more likely to achieve ≥ 90% reduction in PSA (PSA90) by 6 months after initiating treatment than patients treated with abiraterone acetate (P = .016).22 Similarly, a separate comparative study found that patients with mCSPC treated with apalutamide were 56% more likely to achieve PSA90 than patients treated with enzalutamide (P = .014).21
Results from this study should be considered in the context of certain limitations. Given the descriptive nature of this study and the small sample size, no adjustments for potential confounders, formal comparisons, or significance testing were performed. Differences in measured baseline characteristics, such as comorbidities (ie, Quan-CCI), prevalence of visceral metastasis, and resource utilization observed, may explain postindex medical costs for these cohorts. As with all observational studies that use real-world data sources (eg, electronic medical record, claims databases), coding inaccuracies or omissions may exist in the data. Specifically, the selection of patients with mCSPC relied on the provided electronic medical record variables, which may have led to misclassification depending on the inaccuracies or omissions contained in the database. Lastly, given earlier mCSPC indication approval for abiraterone acetate, patients in this cohort were observed with longer follow-up than those treated with apalutamide or enzalutamide.
Conclusion
In this descriptive study of patients with mCSPC, annual all-cause and PC-related medical costs were similar between enzalutamide and abiraterone acetate cohorts, and descriptively lower in the apalutamide cohort. Variations in medical and pharmacy costs observed among the three ARPI agents investigated may reflect how well these agents manage PC. Future work leveraging more recent data and enhanced cohorts will allow for formal comparative analyses evaluating HRU and costs across different ARPIs in this area, which may facilitate a framework for health care value that may be of interest to population health decision makers.
Clinical Pathway Categories: Business + Outcome Measurements
There is a high economic burden of metastatic castration-sensitive prostate cancer (mCSPC) and increased health care resource use (HRU) and costs associated with progression from mCSPC to metastatic castration-resistant PC. Clinical pathways help in tracking and evaluating the total cost of care, components of HRU, and costs of newer therapeutic agents such as androgen receptor pathway inhibitors, particularly in a Medicare-insured population.
Author Information
Affiliation:
1Johnson & Johnson, Horsham, PA, USA; 2Analysis Group, Inc, Montréal, QC, Canada
Funders:
Johnson & Johnson sponsored the study.
Correspondence:
Carmine Rossi, PhD
Manager, Analysis Group, Inc.
1190 Av. des Canadiens-de-Montréal, Suite 1500
Montreal, QC H3B 0G7, Canada
Phone: 514-871-4233
Email: carmine.rossi@analysisgroup.com
Disclosures:
C.R., J.K., L.D., B.M., and D.P., are employed by Analysis Group, Inc, a consulting company that has provided paid consulting support to Johnson & Johnson, which sponsored the study. I.K., S.D., and L.E. are employed by Johnson & Johnson.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
2. Mosillo C, Iacovelli R, Ciccarese C, et al. De novo metastatic castration sensitive prostate cancer: state of art and future perspectives. Cancer Treat Rev. 2018;70:67- 74. doi:10.1016/j.ctrv.2018.08.005
3. Cancer stat facts: prostate cancer. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Accessed October 22, 2024. https://seer.cancer.gov/statfacts/html/prost.html
4. Hormone therapy for prostate cancer. American Cancer Society. Revised November 22, 2023. Accessed October 22, 2024. https://www.cancer.org/cancer/types/ prostate-cancer/treating/hormone-therapy.html
5. Harada K, Shiota M, Minato A, et al. Treatment strategies for metastatic castration-sensitive prostate cancer: From “all-comers” to “personalized” approach. Onco Targets Ther. 2021;14:2967-2974. doi:10.2147/ott.S306345
6. Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76-85. doi:10.1038/ncpuro1296
7. Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32(49):5501-5511. doi:10.1038/onc.2013.206
8. Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer. 2010;17(4):R305-R315. doi:10.1677/erc-10-0187
9. Meagher MF, Salmasi A, Stewart TF. Treatment landscape for metastatic castrate-sensitive prostate cancer: a review. Res Rep Urol. 2023;15:509-517. doi:10.2147/ rru.S398129
10. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. doi:10.1056/NEJMoa1903307
11. Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303. doi:10.1200/ jco.20.03488
12. Erleada. Prescribing information. Janssen; 2024. Accessed October 22, 2024. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ERLEADA-pi.pdf
13. Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022; 40(15):1616-1622. doi:10.1200/jco.22.00193
14. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi:10.1200/jco.19.00799
15. Xtandi. Prescribing information. Astellas; 2023. Accessed October 22, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/213674s010,203415s022lbl.pdf
16. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. May 2019;20(5):686-700. doi:10.1016/s1470-2045(19)30082-8
17. Zytiga. Prescribing information. Janssen; 2024. Accessed October 22, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202379s024lbl.pdf
18. Nubeqa. Prescribing information. Bayer; 2023. Accessed October 22, 2024. https:// labeling.bayerhealthcare.com/html/products/pi/Nubeqa_PI.pdf
19. Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132-1142. doi:10.1056/ NEJMoa2119115
20. Crawford ED, Andriole G, Freedland SJ, et al. Evolving understanding and categorization of prostate cancer: preventing progression to metastatic castration-resistant prostate cancer: RADAR IV. Can J Urol. 2020;27(5):10352-10362.
21. Lowentritt B, Pilon D, Khilfeh I, et al. Attainment of early, deep prostate-specific antigen response in metastatic castration-sensitive prostate cancer: a comparison of patients initiated on apalutamide or enzalutamide. Urol Oncol. 2023;41(5):253. e1-253.e9. doi:10.1016/j.urolonc.2023.03.003
22. Lowentritt B, Pilon D, Waters D, et al. Comparison of prostate-specific antigen response in patients with metastatic castration-sensitive prostate cancer initiated on apalutamide or abiraterone acetate: a retrospective cohort study. Urol Oncol. 2023;41(5):252.e19-252.e27. doi:10.1016/j.urolonc.2023.03.013
23. Kaye DR, Khilfeh I, Muser E, et al. Characterizing the real-world economic burden of metastatic castration-sensitive prostate cancer in the United States. J Med Econ. 2024;27(1):381-391. doi:10.1080/13696998.2024.2323901
24. Kaye DR, Khilfeh I, Muser E, et al. Real-world economic burden associated with disease progression from metastatic castration-sensitive to castration-resistant prostate cancer on treatment in the United States. J Manag Care Spec Pharm. 2024;30(7):684-697. doi:10.18553/jmcp.2024.30.7.684
25. Kaye DR, Khilfeh I, Muser E, et al. Real-world economic burden of metastatic castration-resistant prostate cancer before and after first-line therapy initiation. J Med Econ. 2024;27(1):201-214. doi:10.1080/13696998.2024.2303890
26. Glaus CEG, Kloeti A, Vokinger KN. Defining ‘therapeutic value’ of medicines: a scoping review. BMJ Open. 2023;13(12):e078134. doi:10.1136/bmjopen-2023-078134
27. Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23):2563-2577. doi:10.1200/jco.2015.61.6706
28. Freedland SJ, Ke X, Lafeuille MH, et al. Identification of patients with metastatic castration-sensitive or metastatic castration-resistant prostate cancer using administrative health claims and laboratory data. Curr Med Res Opin. 2021;37(4):609-622. doi:10.1080/03007995.2021.1879753
29. Ryan CJ, Ke X, Lafeuille MH, et al. Management of Patients with metastatic castration-sensitive prostate cancer in the real-world setting in the United States. J Urol. 2021;206(6):1420-1429. doi:10.1097/ju.0000000000002121
30. Trinh QD, Chaves LP, Feng Q, Zhu J, Sandin R, Abbott T. The cost impact of disease progression to metastatic castration-sensitive prostate cancer. J Manag Care Spec Pharm. 2022;28(5):544-554. doi:10.18553/jmcp.2022.28.5.544
31. Appukkuttan S, Tangirala K, Babajanyan S, Wen L, Simmons S, Shore N. A retrospective claims analysis of advanced prostate cancer costs and resource use. Pharmacoecon Open. 2020;4(3):439-447. doi:10.1007/s41669-019-00185-8
32. Freedland SJ, Davis MR, Epstein AJ, Arondekar B, Ivanova JI. Healthcare costs in men with metastatic castration-resistant prostate cancer: an analysis of US Medicare fee-for-service claims. Adv Ther. 2023;40(10):4480-4492. doi:10.1007/s12325- 023-02572-4
33. Wu B, Li SS, Song J, Pericone CD, Behl AS, Dawson NA. Total cost of care for castration-resistant prostate cancer in a commercially insured population and a medicare supplemental insured population. J Med Econ. 2020;23(1):54-63. doi:10.1080/13696 998.2019.1678171
34. US Food and Drug Administration. FDA approves darolutamide tablets for metastatic hormone-sensitive prostate cancer. News release. August 5, 2022. Accessed October 22, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-darolutamide-tablets-metastatic-hormone-sensitive-prostate-cancer


















