Weighing Costs and Side Effects in Psoriasis Management
Psoriasis is a chronic systemic inflammatory autoimmune disease characterized by skin lesions. The most common subtype is plaque psoriasis, which accounts for 80-90% of all cases. Psoriasis is associated with an increased incidence of chronic comorbid conditions, related to the systemic inflammatory nature of the disease. Patients with psoriasis have an increased risk for metabolic syndrome, including obesity, diabetes, and hyperlipidemia, with an increased overall mortality risk in patients with severe psoriasis.
In addition, up to 30% of patients with psoriasis also have psoriatic arthritis, which manifests as generalized fatigue, pain, stiffness, and swelling in and around the joints, potentially resulting in joint destruction and further disability.1
Estimates of psoriasis prevalence in the United States range between 1% and 5%; in 2013, approximately 7.4 million US adults has psoriasis. Approximately 150,000 cases of psoriasis are diagnosed in the United States annually, and the disease results in 3 million visits to
doctors’ offices or hospitals per year.2
Choices in Treatment
Various options are available for the treatment of psoriasis, including topical agents; phototherapies and photochemotherapy; and systemic nonbiologic drugs, such as methotrexate and cyclosporine (both immunosuppressive drugs), acitretin (a second-generation retinoid), and apremilast (an oral small-molecule inhibitor of phosphodiesterase-4). In addition, several systemic biologic therapies are currently approved by the US Food and Drug Administration (FDA) for the treatment of chronic, moderate-to-severe plaque psoriasis, including infliximab, etanercept, adalimumab, ustekinumab, secukinumab, and ixekizumab.1
In a survey conducted in the United States and several other countries, dermatologists reported that, among patients with moderate-to-severe psoriasis, approximately 75%, 20%,
and 20% of patients were receiving topical
therapy, conventional oral therapy, and biologics, respectively.2
The annual costs for select systemic therapies currently approved by the FDA for the treatment of chronic, moderate to severe plaque psoriasis are considerable. The annual wholesale acquisition cost for newer systemic and biologic therapies range from $30,001 to $88,402.2
Larry Hsu, MD, medical director, Hawaii Medical Service Association said, “With 3 branded interleukin (IL)-17 [inhibiting] agents—Consentyx [sekukinumab], Siliq [brodalumab], and Taltz [ixekizumab]—and now Tremfya [guselkumab], an IL-23 inhibitor, health plans are working to pick not only a preferred biological agent for the treatment of psoriasis, but a preferred IL-17 inhibitor and or preferred interleukin inhibitor.”
Cost of Care
A recent US observational study reported the mean 6-month direct costs for patients with moderate to severe psoriasis to be an annual direct cost of $22,582 per patient; in this study, 60% of patients received biologic therapy, with 36% using a self-administered biologic. In a retrospective analysis of a large US health care claims database, the mean
annualized total health care cost for patients with moderate to severe psoriasis receiving biologic therapy between January 2007 and March 2012 was $30,568.
Magellan Rx Management reported that the cost for biologic drugs for psoriasis/psoriatic arthritis more than tripled (230% increase) from $0.17 per member per month in 2012 to $0.57 in 2016.3 The majority of these costs are accounted for by only a few biologic medications (Table).3
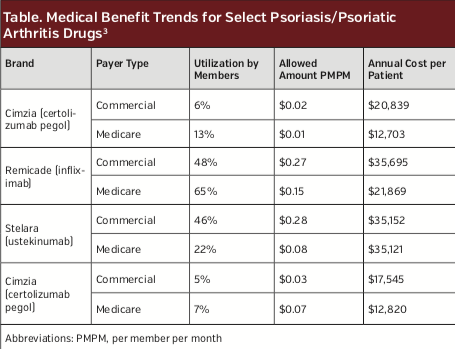
Payers face substantial challenges when considering which psoriasis treatments to include in their plan for reimbursement, particularly in the setting of moderate to severe psoriasis for which biologic or oral therapies may be
indicated. Payers have to balance the potential benefit of treatments with any risk of side effects, as well as take into account the cost of therapy (including the cost of monitoring for side effects and the likely cost of managing disease sequelae in the absence of treatment).2
As a consequence, payers often support a traditional step-wise approach to treatments for psoriasis. Traditional nonbiologic therapies with much lower acquisition costs may berequired as first-line treatments even for patients with moderate to severe psoriasis, for whom some dermatologists think a biologic or a recently approved nonbiologic drug with higher acquisition costs are warranted for initial therapy. ν
References
1. Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-50.
2. Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504-513.
3. Magellan Rx Management. Medical Pharmacy Trend Report. 2017 Eighth Edition.https://www1.magellanrx.com/magellan-rx/publications/medical-pharmacy-trend-report.aspx. Accessed February 12, 2018.












