Therapies for Managing Psoriasis
San Diego— In recent years, an increased understanding of psoriasis pathophysiology has led to the release of new treatment targets. Additionally, how to manage the repercussions of psoriasis treatments on associated comorbid diseases and how therapies for comorbidities impact efficacy and tolerability of psoriasis treatments is of importance. There is a need for collaboration by all healthcare workers, including pharmacists, involved with psoriasis to better manage this patient population.
Psoriasis is the most common, chronic, multifaceted autoimmune disease in the United States affecting between 5.8 and 7.5 million people. The most common type of psoriasis is chronic plaque psoriasis, which affects about 90% of the psoriatic patient population. In the United States, 150,000 to 260,000 new cases of psoriasis are diagnosed each year. Psoriasis is classified as mild (<3% body surface area [BSA] involvement), moderate (3%-10% BSA involvement), and severe (>10% BSA involvement), said Geoffrey C. Wall, PharmD, FCCP, BCPS, CGP, professor of clinical sciences, Drake University College of Pharmacy and Health Sciences, IA, during a satellite symposium at the AMCP Specialty Pharmacy Conference supported by an educational grant from Eli Lilly.
“[With] mild, moderate, and severe [psoriasis it] can be difficult to determine who is right for what treatment—localized versus systemic or phototherapy,” said Dr. Wall, who is also an internal medicine clinical pharmacist, Iowa Methodist Medical Center.
Evolving Targets in Psoriasis
“Our understanding of [psoriasis] is still a work in progress,” said Junko Takeshita, MD, PhD, clinical instructor, department of dermatology, University of Pennsylvania. “We are learning more and more about genetics of psoriasis. We may see some genetically modified treatment for psoriasis down the line, though not right now.”
Biologics
Biologic therapies have been shown to improve the lives of individuals with skin disease. Currently, there are 5 FDA-approved biologics for moderate-to-severe psoriasis: (1) etanercept; (2) inflix- imab; (3) adalimumab; (4) ustekinumab; and (5) secukinumab, according to Dr. Takeshita, who discussed biologic therapies in the pipeline for psoriasis.
The biosimilar infliximab was approved by the European Medicines Agency in 2013. He said several tumor necrosis factor biosimilar trials are underway in the United States for etan-ercept and adalimumab. FDA approval guidelines and regulations on future use of biosmiliars are being hotly debated in the United States. “Realization of the biosimilar promise of decreased cost and increased access may be challenging,” he added.
Other biologics in the pipeline include therapies that selectively block interleukin (IL)-23 (tildrakizumab, guselkumab, and BI 655066), IL-17A (ixekizumab), and IL-17RA (brodalumab). Dr. Takeshita said that ixekizumab and brodalumab may receive FDA approval in 2016. He briefly discussed clinical trial results for ixekizumab. A phase 2, double-blind, placebo-controlled study by Leonardi and colleagues found that the anti-IL-17 monoclonal antibody ixekizumab improved the clinical symptoms of psoriasis as measured by the reduction in Psoriasis Area and Severity Index 75% (PASI 75). At 12 weeks, the percentage of patients with a reduction in the PASI 75 was significantly greater with ixekizumab 150 mg (82.1%), 75 mg (82.8%), and 25 mg (76.7%) than with placebo [N Engl J Med. 2012;366(13):1190-1199].
Dr. Takeshita concluded by offering general impressions of the new biologic therapies. New biologics are more targeted to the skin immune system than to the systemic immune system, which makes efficacy very high and safety profiles very good. It is too early to say how effective they will be for psoriatic arthritis (PsA), which affects approximately 6% to 40% of patients with psoriasis, depending on population. It is also too early to say whether they will improve systematic inflammation and disease cardiovascular risk.
Small Molecule Inhibitors
Emerging targeted therapies for psoriasis include the small molecule phosphodieterase (PDE) 4 inhibitors, Janus kinase inhibitors, and adenosine receptor inhibitors. Apremilast is the first and only PDE 4 inhibitor the FDA approved for moderate-to-severe psoriasis. It is also approved for adults with active PsA. Phase 3 trials of apremilast have shown moderate efficacy for psoriasis with PASI 75 ranging from 21% to 41%, said Dr. Takeshita referencing a study by Kelly and colleagues [Dermatol Clin. 2015;33(1):91-109.] Common side effects the occurred within the first 2 weeks were diarrhea, nausea, and headache. Other common side effects reported were upper respiratory tract infection and unexplained weight loss.
Other Psoriasis Therapies
Common topical therapies used to treat psoriasis include corticosteroids, vitamin D analogs, retinoids (eg, tazarotene), and calcineurin inhibitors (eg, tacrolimus and pimecrolimus). Phototherapy is also another option, but Dr. Wall noted that it is expensive and requires multiple appointments for patients. Furthermore, it is difficult to administer to patients with scalp psoriasis due to the high density of hair follicles as well as the difficulty in avoiding nonaffected areas. Conventional therapies are also available and include methotrexate, acitretin, and cyclosporine. “Methotrexate is not going anywhere and it is effective,” he said, noting that patients need to be taking daily folic acid with it.
Ongoing assessment of therapeutic efficacy and side effects is essential to optimize outcomes when initiating any psoriasis therapy. Available biologics require particularly vigilant monitoring for rare but serious adverse events, according to Dr. Wall.
Psoriasis and Comorbidities
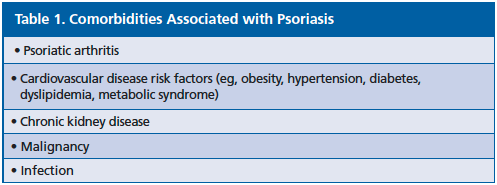
As outlined in the Table 1, psoriasis is associated with various comorbidities. Studies have found that psoriasis is associated with more severe hypertension [PLoS One. 2011;6(3):e:18227]. Another study showed that the prevalence of hypertension increases with greater psoriasis BSA involvement [JAMA Dermatol. 2015;151(2):161-169]. Also the prevalence of metabolic syndrome increases with greater BSA involvement [J Invest Dermatol. 2012;132(3 Pt 1):556-562].
In terms of whether treating psoriasis lowers cardiovascular disease risk, Dr. Takeshita said, “My answer is ‘unsure.’” He said various studies have shown a correlation, while other studies have demonstrated no benefit. He emphasized that there are no guidelines for psoriasis and cardiovascular disease.
Role of Pharmacists
Pharmacists play a critical role in ongoing psoriasis management. Pharma- cists should monitor disease progression such as identifying signs and symptoms of PsA; educate patients about the disease including discussing treatment options; assess treatment responses and adverse effects; and ensure appropriate patient monitoring. For patients prescribed biologic therapy, pharmacists should monitor laboratory tests and vaccinations, according to Dr. Wall, who referenced a study by Ermer and colleagues [J Clin Aesthet Dermatol. 2010;3(8):20-26]. Based on a literature review and package inserts for biologics, the researchers recommended withholding live and live-attenuated vaccinations (eg, varicella, herpes zoster, and inhaled influenza) during biological therapy and to consider routine influenza and pneumococcal vaccination in patients who are at high risk.
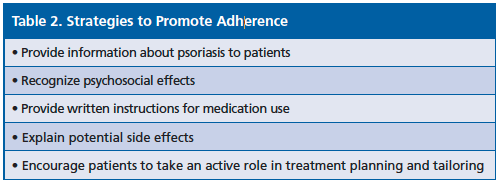
Dr. Wall also emphasized treatment adherence in patients and the pharmacists’ role, offering strategies to promote adherence (Table 2).—Eileen Koutnik- Fotopoulos