Targeting the Human Gut Microbiome to Prevent Degenerative Disease
San Diego, CA—The human gut microbiome may be the solution to prevention and treatment of many of the most common modern degenerative diseases, according to a presentation by David J. Scheiderer, MD, Integrative Psychiatry, Inc. Alzheimer’s disease, anxiety, autism spectrum disorders (ASDs), dementia, depression, diabetes heart disease, HIV, obesity, and Parkinson’s disease, he said, are associated with decreased gut microbial diversity, increased intestinal permeability, dysbiosis, and chronic-low grade inflammation, or “metaflammation.”
Chronic degenerative conditions, including most mental disorders, are on the rise and have overtaken infections as a major cause of death and illness. This is likely due in part to environmental changes, but is also associated with low-grade, chronic, systemic inflammation. Multiple, converging lines of evidence have shown the primary cause of this inflammation may be dysfunction of the “gut-brain axis.” This system-wide inflammation disturbs psycho-neuroimmunological parameters such as neurotransmitters, hormones, growth factors, and immune reactivity.
Dr.Scheiderer explained that individuals may be predisposed to such stress-induced inflammation by negative in utero and early life experiences that alter initial gut colonization. Because the gut ecology is modified, stressors become magnified, stress response is amplified, and corrective homeostatic mechanisms are inhibited and result in chronic sustained inflammation.
Dysbiosis: A Plausible Clue
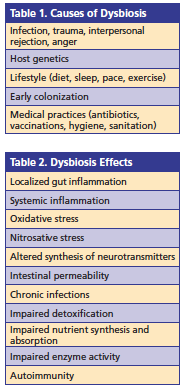 Dysbiosis is the alteration of a microbiome caused by a change in the composition of the microbiota, a change in microbial metabolic activity, and/or a shift in local distribution of the communities of microbes. Dysbiosis provides a plausible clue as to the origin of systemic metabolic disorders encountered in clinical practice that may explain the epidemic of chronic diseases. Causes and effects of dysbiosis can be seen in Tables 1 and 2.
Dysbiosis is the alteration of a microbiome caused by a change in the composition of the microbiota, a change in microbial metabolic activity, and/or a shift in local distribution of the communities of microbes. Dysbiosis provides a plausible clue as to the origin of systemic metabolic disorders encountered in clinical practice that may explain the epidemic of chronic diseases. Causes and effects of dysbiosis can be seen in Tables 1 and 2.
LPS and Acute Anxiety
Lipopolysaccharides (LPS) are structural fragments of the external membrane of gram-negative bacteria. In healthy adults, only miniscule amounts of LPS are found in the blood, indicating intact intestinal barrier function. However, even at low levels, he continued, LPS administration causes acute anxiety, depressed mood, cognitive blunting, and increased visceral pain sensitivity. Higher circulating LPS levels are associated with abdominal obesity, metabolic syndrome parameters (elevated insulin, triglycerides, and total cholesterol), and diabetes. Circulating LPS causes and perpetuates increased permeability of the intestinal and blood-brain barriers.
Disturbances and ASD
Subsets of children with ASD display gastrointestinal abnormalities, including increased intestinal permeability, or “leaky gut,” Dr Scheiderer said. It is believed that autism may be, in part, a disease involving the gut that impacts immune, metabolic, and nervous systems, and that microbiome-mediated therapies may be a safe and effective treatment for ASD. Considerable evidence has linked various diets to gut microbiota changes and subsequent health effects. For example, diets containing noncaloric artificial sweeteners have been linked to dysbiosis and glucose intolerance. In patients with ASD, diets free of gluten and casein may treat some of the core and behavioral symptoms these patients display by having a favorable influence on gut microbiota populations and intestinal barrier function.
Administering probiotic bacteria to address the changes in microbiota may have the ability to reduce inflammation, restore epithelial barrier function, and possibly ameliorate the behavioral symptoms associated with ASD. Clinical studies linking the therapeutic impact of probiotics on neuropsychological disorders are limited and in the early stages, however, results thus far support those from preclinical studies and suggest the modulation of microbiota may indeed be targeted for their therapeutic potential in neuropsychological disorders.
Non-Celiac Gluten Sensitivity
Non-celiac gluten sensitivity (NCGS) has been linked to many extraintestinal systemic symptoms, including anxiety, “brain fog,” depression, eczema/rash, fatigue, headaches, and pain. Addition- ally, NCGS increases vulnerability for dementia. Dr Scheiderer indicated that evidence has shown that as many as 57% of individuals with neurological dysfunc- tion of unknown origin test positive for anti-gliadin antibodies. One study showed that the prevalence of anti-gliadin antibodies was significantly higher in people with schizophrenia compared to the general population; another study showed patients with recent onset schizophrenia had increased levels of IgA and IgG antibodies to gliadin compared with a control population.
There is also mounting evidence that Parkinson’s disease is not only a brain disease but also a digestive disorder, as expression of the tight junction (TJ) protein occludin is decreased in those with Parkinson’s disease.
HIV and Gut Microbiota
The human immunodeficiency virus (HIV) is capable of drastically altering the immune system and the gastrointestinal environment, leading to significant changes to the gut microbiota and mucosal permeability that results in microbial translocation from the gut extending to the blood and even the brain. This microbial translocation and subsequent downstream effects create a self-sustaining feedback loop that enhances HIV disease progression and constitutes a vicious cycle of inflammation and im- mune activation combining viral and bacterial factors. An understanding of this self perpetuating cycle could be a key element in developing new therapies that are aimed at the gut microbiota and its fallout after infection, Dr Scheiderer said.
Weight Gain
Certain agents, such as olanzapine and risperidone, induce alterations in the gut microbiota of rats when administered chronically, with other metabolic abnormalities occurring at the same time, including weight gain, increased visceral fat, and a proinflammatory phenotype. As such, the gut microbiota represent both a biomarker and a potential therapeutic target for obesity, metabolic disease, and drug-induced weight gain.
Inflammatory Inducers
Some of the pro-inflammatory inducers of metaflammation include aging, inactivity, obesity, smoking, sleep deprivation, stress, anxiety, depression, air pollution, and a high omega 6 to omega 3 intake ratio. Anti-inflammatory inducers include exercise, restricted energy intake, fish/ fish oils, and weight loss. Dietary antioxidant sources such as coffee, cocoa, green tea, blueberries, and curcumin have all been linked to a lowered risk of depression and/or cognitive decline. These agents have also been shown to have beneficial effects on gut microbiota.—Mary Mihalovic













