Management of Rheumatoid Arthritis
 Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease that affects an estimated 1.5 million patients in the United States,1 making it the most common autoimmune inflammatory arthritis in adults. It is characterized by inflammation of the synovial membranes and leads to progressive erosion and destruction of cartilage and bone tissue in the affected joints.
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease that affects an estimated 1.5 million patients in the United States,1 making it the most common autoimmune inflammatory arthritis in adults. It is characterized by inflammation of the synovial membranes and leads to progressive erosion and destruction of cartilage and bone tissue in the affected joints.
RA has a significant negative impact on the ability to perform daily activities and on health-related quality of life, conferring a substantial health care and economic burden. Costs attributable to RA are expected to further increase as the population ages and the prevalence of obesity and physical inactivity increases.1
Medication Management of RA
Treatment options for RA include traditional disease-modifying antirheumatic drugs (DMARDs), biologic DMARDs, Janus kinase (JAK) inhibitors, and glucocorticoids. Common DMARDs include methotrexate (Trexall, Otrexup, Rasuvo), leflunomide (Arava), hydroxychloroquine (Plaquenil) and sulfasalazine (Azulfidine).2 These drugs can slow the progression of RA and spare the joints and other tissues from permanent damage.
The newer class of DMARDs are known as biologic response modifiers, which block immune system chemical signals that lead to inflammation and joint/tissue damage. These include the tumor necrosis factor inhibitors (TNFis) abatacept (Orencia), adalimumab (Humira), anakinra (Kineret), certolizumab (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade), rituximab (Rituxan, MabThera), and tocilizumab (Actemra).2
People who cannot be treated with methotrexate alone may be prescribed a JAK inhibitor such as tofacitinib (Xeljanz).
Sarilumab (Kevzara) was approved May 2017 by the FDA to treat adults with moderate to severe active RA who do not respond well to or have intolerance to DMARDs, such as methotrexate.2
For patients with symptomatic early RA, treatment is typically initiated with nonsteroidal antiinflammatory drugs and/or low-dose glucocorticoids. The American College of Rheumatology (ACR) guidelines recommend that a traditional DMARD, preferably methotrexate, be initiated as quickly as possible after diagnosis, with monotherapy preferred over double or triple therapy.3 If disease activity remains moderate or high despite methotrexate or other traditional DMARD treatment, a biologic agent is typically added to the regimen. ACR recommends a TNFi be used over tofacitinib. If disease activity is not adequately controlled, it is common to switch to either another biologic agent with a different mechanism of action or from one TNFi to another.3
In the United States, intraclass switching of TNFis is common within clinical practice. In a survey study, more than 94% of rheumatologists reported switching patients from one TNFi to another because of inadequate response or side effects.1
Payer Management Strategies
The introduction of targeted biological therapies has revolutionized the management of RA. Following treatment with these therapies, many patients experience significant improvements in different aspects of their disease, including symptom burden, work productivity, and quality of life. However, the costs of these therapies are relatively high.
“From a pharmacy benefits perspective, the biggest challenge is managing specialty medications," Catherine Cooke, PharmD, BCPS, PAHM, president of PosiHealth, Inc, told First Report Managed Care. "The overall health gains from these medications depend on real-world patient experience, and for patients that do benefit, the gains may be difficult to quantify economically. The health gain may not be considered in silo pharmacy budgets. And, even for payers with a more integrated perspective, some of the gain may be more important for function, and potentially for employers.”
Indeed, the financial burden on health care systems due to biologic therapies is considerable. RA represented the fifth highest drug spend category for Medicare and for commercial payers in 2016, at $1.17 per member per year (PMPM) and $1.65 PMPM, respectively.4
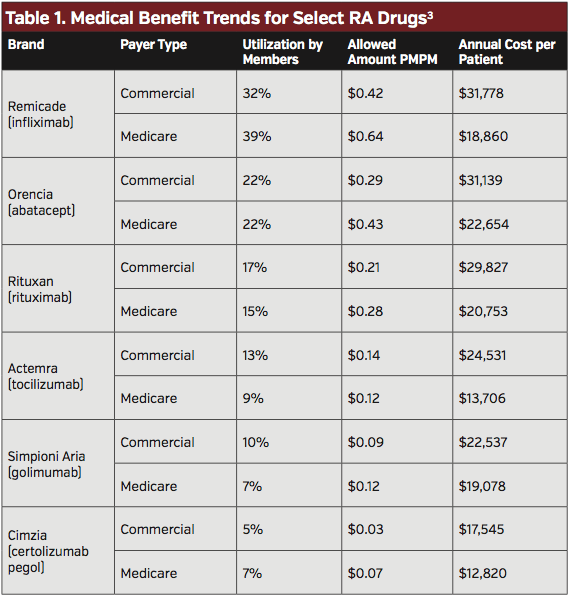
Lack of patient access to effective treatment remains a concern. Recent trends in utilization of biologics for the treatment of RA show that Remicade consistently had the most utilization, both for patients covered by commercial payers and those covered by Medicare (Table 1).4 Orencia had the second highest utilization, followed by Rituxan. In terms of costs, Remicade also accounted for the highest PMPM spend for both commercial and Medicare beneficiaries with RA when compared with other biologics for RA.4
“The biggest challenge in RA is the movement towards oral drugs and the over-utilization of these treatments,” Arthur F Shinn, PharmD, FASCP, Managed Pharmacy Consultants, LLC, told First Report Managed Care. “Because these are more expensive, we have implemented step therapy requirements. However, there has been a lot of resistance to this, and we’ve received an influx of appeals from providers who feel strongly that these treatments are superior.”
Managing the costs for these treatments is a significant area of focus for payers. Most biologic therapies, and some of the newer nonbiologic agents, are effective in multiple autoimmune (and nonautoimmune) diseases, but have different dosage regimens across indications, which can present a challenge to payers. Payer formulary strategy and utilization management are frequently based on a collective therapeutic review for all indicated autoimmune diseases. Such reviews are often based on the autoimmune disease with the largest patient population, which is RA. This approach may result in a formulary that many limit or even preclude access to some treatment options for patients with other conditions, such as moderate to severe psoriasis.5
"The same drugs are used across the disease states (or at least in the contract market baskets if they have fewer indications), and rebate contracts are by drug, not by disease state," a medical executive of a pharmacy services management company told us. "This limits our flexibility to have cost-effective formularies that may differ by disease state; for example, the most cost-effective psoriasis drugs may not be the most cost-effective RA drugs."
Impact of Biosimilars
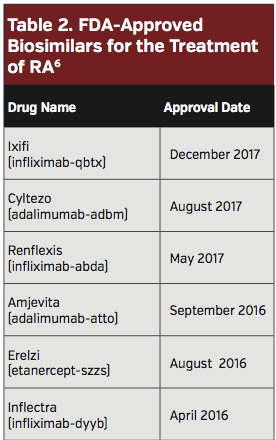
Several biosimilars have been approved for RA indications (Table 2). With three biosimilar versions of Remicade gaining FDA approval, significant change is expected in this market.4 Biosimilars have the potential to significantly reduce expenditures secondary to the use of biologic medications. There are growing data demonstrating similar clinical efficacy, immunogenicity, and safety of each of the approved infliximab and adalimumab biosimilars.
With the introduction of biosimilar agents covered on the medical benefit, payers have a greater ability to implement management strategies to drive a lowest net cost product in key therapy classes.
“Health plans are still developing strategies to favor the biosimilars that are already approved and to be approved for RA; eg, for infliximab and adalimumab,” said Larry Hsu, MD, Medical director, Hawaii Medical Service Association, in an interview with First Report Managed Care. Meanwhile, the companies producing the originator drugs are countering these efforts by more aggressive contracts for their agents, delaying the onset of biosimilars via legal challenges, and, in some cases, reformulating their originator drug to prolong the use of their brand.”
References
1. Claxton L, Jenks M, Taylor M, et al. An economic evaluation of tofacitinib treatment in rheumatoid arthritis: modeling the cost of treatment strategies in the United Sates. J Managed Care Specialty Pharm. 2016;22(9):1088-1102.
2. American College of Rheumatology. Rheumatoid Arthritis. ACR website. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis. Updated March 2017. Accessed September 29, 2018.
3. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthr Care Res. 2016;68(1):1-25.
4. Magellan Rx Management. Medical Pharmacy Trend Report. 2017 Eighth Edition. Available at: https://www1.magellanrx.com/magellan-rx/publications/medical-pharmacy-trend-report.aspx. Published February 12, 2018. Accessed September 29, 2018.
5. Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benifits. 2016;9(9):504-513.
6. FDA. Biosimilar Product Information. FDA-Approved Biosimilar Products. FDA.gov website. https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm580432.htm. Updated July 20, 2018. Accessed September 29, 2018.










