ADVERTISEMENT
IMS Health Identifies Potential $213.2 Billion Savings in Healthcare Costs
Tampa—In June 2013, the IMS Health Institute for Healthcare Informatics issued a report that mentioned the United States’ healthcare system could save $213.2 billion in annual healthcare costs if medications were used more responsibly. The researchers suggested that major opportunities could improve adherence and rely more on evidence-based treatments. They also mentioned savings could be found by focusing on antibiotic misuse, medication errors, suboptimal use of generic drugs, and mismanaged polypharmacy for the older population.
Douglas Long, vice president, industry relations, IMS Health, Inc., discussed the report as well as other major recent industry trends during a session at the AMCP meeting.
“There are a number of actions that could be taken immediately to seize this opportunity,” Mr. Long said. “We would not only increase the value of medicines, we would have an improvement in outcomes and declined healthcare costs.”
Of the $213 billion in potential savings, Mr. Long said $140 billion would be saved in hospitals, and there would be 10 million fewer hospital admissions. People who now misuse medications or take them incorrectly typically end up in hospitals, where the cost to treat patients is higher than in other settings. In 2013, office visits decreased 0.9% but emergency room admissions increased 5.8%. IMS Health found that there are 78 million unnecessary outpatient visits, 246 million unwarranted prescriptions, and 4 million needless emergency room visits.
To achieve the savings, IMS Health recommended the following: (1) pharmacists have an increased role in medication management; (2) older patients undergo medical audits; (3) mandatory reporting of antibiotic use; (4) encourage a “no blame” culture towards error reporting; and (5) support for targeted disease management programs for prevalent, noncommunicable diseases, such as heart disease, stroke, cancer, and diabetes. With the introduction of accountable care organizations and other new delivery systems, physicians will work closer with pharmacists, nurses, and other healthcare professionals and will have an incentive to keep costs down.
“To be successful in this requires collaboration,” Mr. Long said. “The best thing about healthcare reform is there is more skin in the game for everybody. If [patients] are not compliant and end up back in hospitals, people are going to be penalized for that. You have more of a vested interest on all of these players in the areas of avoidable costs.”
Mr. Long mentioned a small percentage of people are driving the high healthcare costs. According to IMS Health data from June 2012, 1% of people in the United States accounted for 26.1% of healthcare spending and 20% accounted for 80.8% of the spending. The top 1% spent at least $48,735 in healthcare costs and included people who use specialty or orphan drugs, have chronic conditions, are not adherent to their medications, and spend time in hospitals and emergency rooms.
In the United States, spending on pharmaceuticals accounts for approximately 10% of the healthcare spending, while hospital care represents 30% and physicians and clinics account for 20%, according to Mr. Long. He noted that people are living longer and developing chronic disease at an earlier age, so they will be on medications for a long time and will be using more specialty and orphan drugs, driving costs even higher. “Cost containment will be with us in healthcare for as long as we are all alive,” Mr. Long said.
Overview of 2013 Prescription Spending
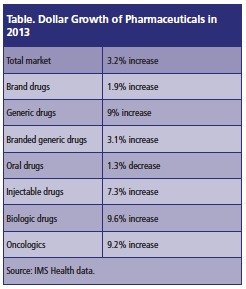
In 2013, prescription growth in the United States increased 1.4%, higher than the 1% growth in 2012. Mr. Long said the major reason for the increase was that there was a strong flu season during the first quarter of 2013. Meanwhile, prescription sales in terms of dollar growth increased 3.2% in 2012, a year after they were down in 2012 for the first time in >50 years. The increase in 2013 was mainly due to growth in generic drug and specialty drug spending (Please see Table below).
The 2012 decrease was attributed to several blockbuster drugs losing their patent protection, including atorvastatin, quetiapine fumarate, and montelukast sodium. The largest patent expiration in 2013 occurred in December when duloxetine lost its protection. However, Mr. Long noted that only 4 of the 12 companies that were approved to sell generic versions of duloxetine did so because of shortages of an active pharmaceutical ingredient. Thus, the price of generic duloxetine was only 20% lower than the brand version compared with the usual discount of 80% to 90%, according to Mr. Long.
IMS Health data showed the 10 largest therapeutic classes accounted for $176.7 billion in spending in 2013, and the top 2—oncology and diabetes—each increased >20% from a year earlier. Four of the top 10 largest therapeutic classes—oncology, autoimmune disorders, HIV, and multiple sclerosis—are considered specialty areas and each increased at least 10% from 2012.
IMS Health defines specialty pharmaceuticals as medicines that treat specific, complex chronic diseases with at least 4 of the following attributes: (1) initiated only by a specialist; (2) generally not oral; (3) requires special handling, unique distribution, high expense, and warrants intensive patient counseling; and (4) requires reimbursement assistance. Mr. Long said more drugs in the future will be administered orally and not require special handling.
For 2014, Mr. Long said the most closely watched specialty class is hepatitis C. Late in 2013, the FDA approved sofosbuvir and simeprevir to treat the disease. The drugs showed marked efficacy improvements and fewer side effects than previous options. They are now widely used, although they are expensive. For instance, sofosbuvir costs $84,000 for a 12-week regimen, leading payers and even lawmakers to contemplate the drug’s cost. Still, Mr. Long expects spending on specialty pharmaceuticals to continue to grow for the foreseeable future as more drugs get approved and patients have more options.
Healthcare Trends in 2013 and Beyond
As healthcare costs grow and employers worry about the Patient Protection and Affordable Care Act, there is growth in private health insurance exchanges, in which companies provide their employees with money each year to purchase coverage in online marketplaces. Mr. Long described the emergence of private exchanges as companies “offloading the liability for health insurance to a third party” and cited IBM’s decision in September to move >100,000 retirees from their company-sponsored plan to a private exchange. Retirees who are >65 years of age now must choose a Medicare plan in an exchange run by Towers Watson & Co.
Time Warner, Inc., also had their retirees switch to private exchanges. Walgreen, Co., joined Sears Holding, Corp., and Darden Restaurants, Inc., as companies forcing employees to purchase insurance through an exchange run by Aon Plc. The companies said the exchanges would provide more options for retirees at lower costs, while the companies would not have to manage their healthcare and would have fixed costs. “I think [private exchanges] will grow,” Mr. Long said.
In 2013, there were also the formation of global purchasing alliances through acquisitions and partnerships between corporations. McKesson, Corp., purchased European drug retail rival Celesio AG to create a large distribution group, while Walgreen, Co., and Alliance Boots GmbH bought a stake in and signed a 10-year contract with AmerisourceBergen, a large drug distribution company. AmerisourceBergen will now handle most of the distribution for Walgreen, Co.
In addition, the safety problems associated with compounding pharmacies that mix individual ingredients of medications and personalize them for patients were another major issue in 2013. After a fungal meningitis outbreak occurred in a compounding pharmacy in Massachusetts related to steroid pain injections, the FDA found similar issues in dozens more compounding pharmacies.
President Barack Obama signed a bill into law in November 2013 that gave compounding pharmacies the option to register with the FDA, pay user fees, report data, and submit to inspections. Although compounding pharmacies are not required to register with the FDA, those that do are hoping the safety and quality checks will help them attract customers who are worried about contracting meningitis or have other concerns.