Emerging Evidence and Future Direction of Chemoprevention in Pancreatic Cancer
Washington, DC—Chemoprevention, the application of drugs to slow or reverse the process of carcinogenesis, is typically used to target populations with preneoplastic lesions such as ductal in situ carcinoma and familial adenomatous polyposis.
It also can be used in healthy patients at high risk of developing malignancies because of genetic syndromes or specific medical conditions, as described by Florencia McAllister, MD, assistant professor, department of clinical cancer prevention, MD Anderson Cancer Center, University of Texas, during a session on chemoprevention of pancreatic cancer at Digestive Disease Week.
Populations targeted for chemoprevention of pancreatic cancer include patients with premalignant lesions (such as pancreatic intraepithelial neoplasias, intraductal papillary mucinous neoplasms, and possibly cysts); high-risk healthy patients with genetic syndromes (germline breast cancer 2 and family history); and those with new onset diabetes of whom 1% will get a diagnosis of pancreatic ductal adenocarcinoma in the first 3 years of diabetes diagnosis, said Dr McAllister.
Critical to chemoprevention of pancreatic cancer is to offer preventive agents at the right time, in the right patients. “There is a window of opportunity for pancreatic cancer prevention,” said Dr McAllister, adding that selection of high-risk patients for clinical trials is key to studying chemoprevention in this setting. For high-risk, healthy patients, she emphasized that chemoprevention agents must have a low toxicity profile.
Distilling the Current Evidence
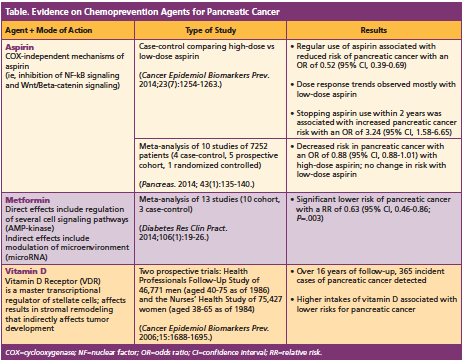
The bulk of Dr McAllister’s presentation focused on the current evidence on various agents used for chemoprevention for pancreatic cancer. She focused on 3 main agents that have been looked at in this setting: (1) low-dose aspirin and high-dose aspirin; (2) metformin; and (3) vitamin D (Table), emphasizing that all epidemiologic data to date on these agents is limited and the agents are not currently used as chemoprevention but as primary prevention. However, she said that chemoprevention trials are possible “as we continue to refine the high-risk population.”
Future Directions
Immunoprevention is an emerging field of investigation in this setting, said Dr McAllister, who emphasized this as an area of high potential. To date, preclinical studies are underway looking at interleukin (IL)-17 monoclonal antibodies.
Along with further investigation into chemoprevention agents for and immunoprevention of pancreatic cancer, intermediary/surrogate markers for chemoprevention responses are also needed because it is challenging to detect premalignant lesions in the field.—Mary Beth Nierengarten