Effect of Medicare Part D and Generics on Breast Cancer Treatment
In the United States, breast cancer is the second most common cancer diagnosis and the second most fatal form of cancer among women. Endocrine therapy agents, including aromatase inhibitors (AIs) and tamoxi- fen, have shown effectiveness in treating breast cancer. Ongoing trials have shown that tamoxifen reduced breast cancer recurrence by 47% in postmenopausal women with hormone receptor positive (HR+) disease. Recent trials have demonstrated AI agents further reduced the recurrence of breast cancer by 30% to 50% compared to tamoxifen among postmenopausal women with HR+ disease, making AI therapy the most effective breast cancer adjuvant endocrine therapy.
Since 2005, the American Society of Clinical Oncology has recommended that postmenopausal women with HR+ disease receive AI therapy or AI therapy with tamoxifen. The high cost of AI agents prevents many breast cancer patients from receiving AI therapy. A recent study compared the costs of AI therapy to tamoxifen therapy during the first 5 years after Medicare Part D was implemented. The researchers also looked at the fluctuation of cost that occurred when generic alternatives were introduced to the market [Springerplus. 2015; DOI:10.1186/ s40064-015-0827-8].
Researchers used the Centers for Medicare & Medicaid Services (CMS) Web site to collect data available on each state’s Medicare Part D plan. They identified deductible amounts, monthly drug premiums, and specific drug costs for the 3 primary AI agents and tamoxifen; (1) anastrozole; (2) exemestane; and (3) letrozole. Annual drug costs were defined as the cost to a Medicare Part D beneficiary who took the recommended dosage of the respective agent for an entire year.
According to the CMS Web site, in 2007, only 9 states offered plans in which the 3 AI agents resulted in no cost to Medicare Part D beneficiaries once the deductibles were met. Tamoxifen was fully covered in at least 1 plan in each state from 2007 to 2011. The generic form of tamoxifen was available to Medicare Part D beneficiaries from 2007 to 2011, according to the CMS Web site. Generics were not available for the 3 AI agents until 2011.
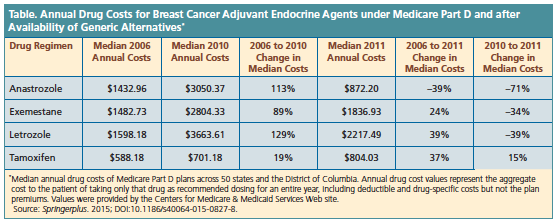
The analysis revealed a sharp in- crease in median state cost of the 3 AI inhibitors under Medicare Part D in 2006 to 2010. Anastrozole increased 113%, from $1433 to $3050; exemes- tane increased 89%, from $1483 to $2804; and letrozole, increased 129%, from $1598 to $3664. Tamoxifen saw a 19% increase in median state cost from $588 to $701 (Table). This resulted in a median annual out-of-pocket costs of $3050, $2804, $3664, and $701 for anastrozole, exemestame, letrozole, and tamoxifen, respectively, in 2010.
When generics became available for the 3 AI inhibitors in 2011, the AI inhibitors decreased in cost while tamoxifen increased. From 2010 to 2011, median state cost for anastrozole was –71%, exemestane was –34%, letrozole was –39%, and tamoxifen was 15%. The median annual costs in 2011 were $872, $1837, $2217, and $804, respectively. From 2006 to 2010, the AI agents saw an increase in annual cost from 89% to 129% for Medicare Part D beneficiaries.
When the generic AI agents became available in 2011, Medicare Part D costs decreased, although it was a private sector event unrelated to Medicare Part D. A limitation of the study is the possibility that the information gathered from the CMS Web site may have had some inaccuracies. The re- sults demonstrate that Medicare Part D may not increase accessibility to treatments; it may be the availability of generics that improves access for beneficiaries.—Melissa D. Cooper