AMCP: Qualified Health Plans: Ratings, Restrictions, and Requirements for 2016 and Beyond
Qualified Health Plans: Ratings, Restrictions, and Requirements for 2016 and Beyond
What kind of prescription drug benefit and quality oversight changes can you expect within the federal health exchanges in 2016, and how will they affect formulary decisions? What proposed requirements are on the horizon for 2017? Two experts provided the answers during AMCP Nexus 2015.
Last winter, CMS submitted its final letter outlining the 2016 prescription drug requirements to insurers in the federally facilitated marketplace. Not only did the 67-page letter include the newly introduced requirements for the 2016 plan year, it also included updates from previous years and some potential requirements for 2017.
The 2016 requirements for qualified health plan (QHP) issuers includes specific conditions (Table 1). In addition to requiring that formularies be available via PDF or as easily searchable documents, QHP plans must make their formulary drug lists and network provider lists available in a machine-readable format. This will allow for increased transparency when data is stored together by third-party vendors.
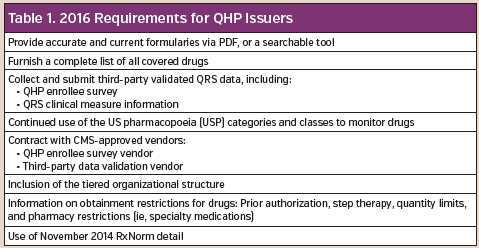
These vendors develop software that gives them access to information so the developers can create new tools for comparison purposes. According to the Department of Health and Human Services, all information should be available in the JavaScript Object Notation (JSON) machine-readable format (Table 2).

As detailed by Deb Schering Sternaman, PharmD, senior director, formulary services, OptumRx, when the requirements for 2015 were released in 2014, there were additional demands with respect to how quickly an expedited review needed to be completed. Now, there are additional restrictions, “much like we have in Medicare Part B where any type of determination for an exception needs to be back in 72 hours even if it’s not expedited,” she said.
Once a product is approved, the drug becomes exchange eligible for the remainder of time a member is on the plan. If the drug is denied approval, a request for an independent review must be submitted.
In order to guarantee non-discrimination in the QHP prescription drug benefit design, CMS reviews each formulary drug list to ensure there is no discrimination in the QHP prescription drug benefit design. CMS performs two types of reviews: (1) a formulary outlier review that identifies reasons why a category or class may be an outlier; and (2) a prescription drug review that analyzes the availability of covered drugs recommended by guidelines for specific disease states. In addition, it ensures there is a sufficient supply of correct drugs with no global restrictions and that various levels of coverage are available.
“There has to be an attestation that there isn’t discrimination against any race, gender, etc.,” said Dr Sternaman. “We’ve been seeing this pop-up a lot in the health care reform required preventive drug categories. There’s a lot of news about transgender individuals and putting gender restriction on things, and that is opening the door for discrimination. Many QHPs are removing restrictions across the board [to eliminate accusations of discrimination].”
Numeric score, global rating, and star rating are required for each quality rating system hierarchy component. The global star rating is based on a 5-point scale. Beginning in the fall of 2016, the marketplace is demanding that these global ratings for all QHP issuers be displayed on their websites.
“Think of it as a hotel rating system,” explained Carolyne Wolf, PharmD, director, government programs quality initiatives, OptumRx. “The more stars, the better the quality.” CMS is providing an easy way to identify which plans have the highest quality.
While the 2017 requirements are subject to change, CMS’s letter did detail some possible requirements in relation to the membership standards.
Specifically:
- There must be a diverse range of clinical specialties to meet enrollees potential needs.
- Health care providers, practicing pharmacists, and practicing physicians must be licensed to prescribe medications.










