Addressing Treatment and Prevention of HIV
HIV is still a major public health problem in the United States. Data from the Centers for Disease Control and Prevention (CDC) estimate that 1.1 million persons in the United States are currently living with HIV,1 including an estimated 15% of individuals with the disease who are unware of their infection.2 More than 700,000 individuals have died of AIDS since the first cases were reported in 1981. The annual number of new HIV diagnoses has decreased slightly in recent years, from about 41,200 new cases in 2012 to 38,300 in 2017, with more diagnoses among men compared with women (80% vs 19%).3
Treatment of HIV
HIV is treated using antiretroviral therapy (ART), which typically combines 3 or more ARTs to prevent HIV replication and disease progression and reduce the risk of transmission.4 Patients are generally initiated on a regimen that includes 2 nucleoside reverse transcriptase inhibitors (NRTIs) and one agent from another class of ART drugs (either a protease inhibitor (PI) or an integrase strand transfer inhibitor (InSTIs).5 Over the past 30 years, ARTs for treating HIV infection have become more effective, safer, and more convenient, leading to increased survival and improved quality of life.6
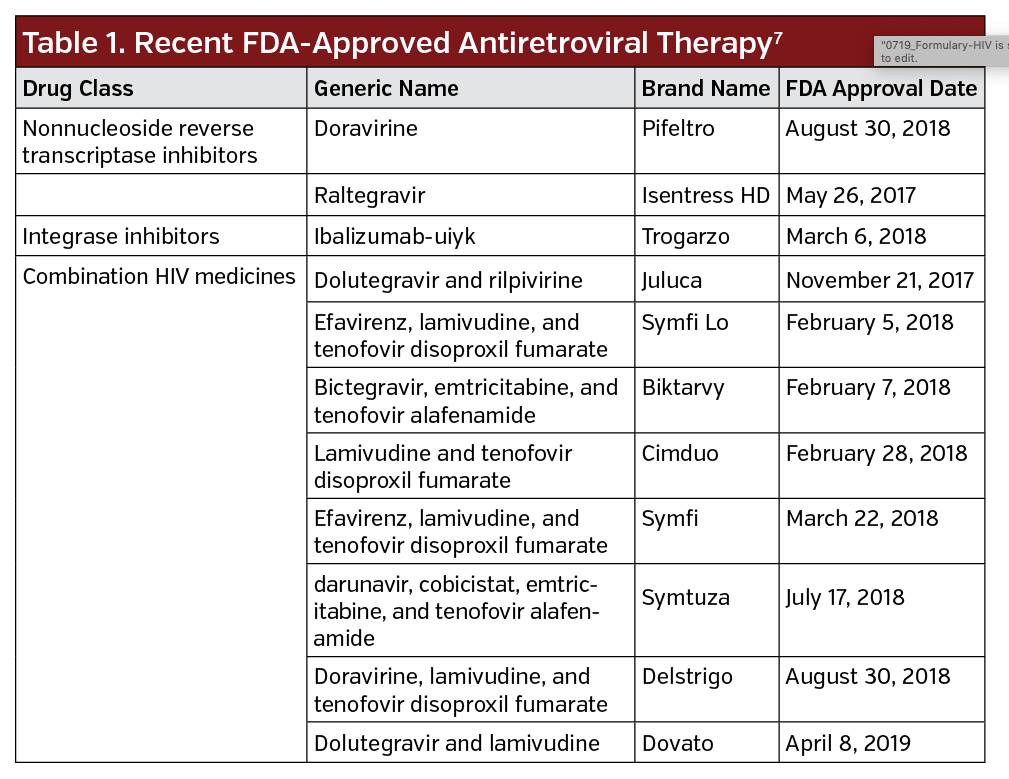
Recent FDA-approved ARTs are listed in Table 1, with the FDA approving eight combination HIV medicines since 2017. Combination HIV medicines contain two or more HIV medicines from one or more drug classes.7 Despite more than 30 FDA-approved drugs, “the pipeline for investigational antiretroviral drugs for treating HIV infection remains full,” said Roy Gulick, MD, MPh, Rochelle Belfer Professor in Medicine at Weill Cornell Medicine in New York, in a Perspectives article published in Investigational Antiretroviral Treatments.6 New agents include compounds in existing classes—NRTIs, nonnucleoside reverse transcriptase inhibitors, PIs, and InSTIs—as well as new classes of HIV entry inhibitors.
Adherence
Adherence to effective ART is essential to achieving durable virologic suppression and minimize drug resistance. In patients with HIV, adherence to ART therapy ≥95% is usually considered necessary for optimal ART efficacy.4 Many factors can negatively affect medication adherence among individuals living with HIV, such as the complexity of the regimen (eg, pill burden and dosing frequency), concerns about side effects, overall mental or physical health, and sociodemographic variables.8
In a retrospective longitudinal study using Medicaid data, researchers found nonadherence to ARTs was observed in 60% to 80% of Medicaid patients and was associated with incremental health care resource utilization and costs. Patients with suboptimal adherence had higher total numbers of days spent in the hospital, total number of long-term care admissions, total medical costs, and inpatient costs. Predictors of adherence included age, insurance type and coverage, previous ART treatment, and HIV symptoms.4
“These findings are of concern, as nonadherence to antiretrovirals remains a significant cause of virologic failure,” concluded the researchers. “Clinicians may consider treatment options that may improve adherence and reduce the risk of drug resistance in patients at risk of virologic failure.”4
PrEP for At-Risk Groups
While great strides have been made in treating people with HIV over the past few decades, the US Preventive Task Force (USPSTF) is strongly recommending preexposure prophylaxis (PrEP) for anyone at risk to help prevent the spread of the disease.9
- Among its recommendations, USPSTF found:
- Convincing evidence that PrEP is of substantial benefit in decreasing the risk of HIV infection in persons at high risk of HIV acquisition
- Convincing evidence that adherence to PrEP is highly correlated with its efficacy in preventing the acquisition of HIV infection
- Adequate evidence that PrEP is associated with small harms
- Adequate epidemiologic data on risk factors that can be used to identify persons at high risk of acquiring HIV infection
Table 2 outlines individuals who should be considered for PrEP. The task force also recommends that clinicians consider PrEP as an option to reduce the risk of HIV acquisition in individuals who use condoms inconsistently, while continuing to encourage and support consistent condom use.
 The new recommendations are consistent with 2017 guidelines from the CDC, which also recommended PrEP for people at risk of infection.10
The new recommendations are consistent with 2017 guidelines from the CDC, which also recommended PrEP for people at risk of infection.10
PrEP is a combination pill of two antiretroviral medications—emtricitabine and tenofovir disoproxil fumarate (Truvada)—that work to stop HIV from multiplying after a person is exposed to it. The once-daily pill was approved by the FDA in 2012. “However, several studies reviewed by the USPSTF found that tenofovir disoproxil fumarate alone was also effective as PrEP, and CDC guidelines note that, given these trial data, tenofovir disoproxil fumarate alone can be considered as an alternative regimen for high-risk heterosexually active men and women and persons who inject drugs,” according to the recommendation statement.9
In an interview with JAMA, John W Epling, MD, MSEd, task force member and coauthor of the statement, noted that PrEP has not really “caught on, and one of the purposes of this recommendation is to get the news out [to] primary care that this a very effective intervention.”
PrEP is currently not used in many individuals at high-risk of HIV infection. CDC data estimates that approximately 1.2 million individuals were eligible for PrEP in 2015,11 and a recent study by Sullivan et al that looked at the annual prevalence of PrEP use estimates that 100,282 individuals were using PrEP in 2017.12
“We are encouraging primary care providers to read and understand our recommendation and strongly consider talking with their patients first about certain behaviors such as sexual and drug use behaviors. If the clinician finds the patient is engaging in these high-risk behaviors to begin the discussion about offering PrEP,” said Dr Epling.
PrEP is highly effective. Dr Epling said that on average in the all studies the task force looked at it was about 73% effective. “The most important thing to think about is the effectiveness depends greatly upon adherence.”
The recommendation also addressed potential harms associated with PrEP, which include increased risk of renal adverse events (primarily grade 1 or greater serum creatinine elevation) and nausea. “The harms are relatively small compared to the potential benefits, and [clinicians] should feel comfortable prescribing PrEP and monitoring for nausea and kidney issues on a regular basis,” said Dr Epling.
The task force also addressed research gaps. Research is needed to develop and validate tools that are highly accurate for identifying persons at high risk of HIV acquisition who would benefit from PrEP. “Risk assessment categories are useful and will help patients and clinicians to further refine the conversation around those health-risk behaviors.” said Dr Epling.
Research is needed on different drug regimens and dosing strategies for PrEP, as well as factors associated with adherence to PrEP and methods to increase uptake and adherence, especially in populations with lower use of and adherence to PrEP. Additionally, trials or demonstration projects of PrEP in US populations of heterosexual persons, persons who inject drugs, and transgender women and men are needed to better quantify effectiveness in those populations. Research is needed to determine whether the use of PrEP is associated with an increased risk of other sexually transmitted infections. Also, research is needed on the long-term safety and effectiveness of PrEP.9
References
1. Centers for Disease Control and Prevention (CDC). Estimated HIV Incidence and Prevalence in the United States, 2010-2016. CDC website. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-1.pdf. Published February 19, 2019. Accessed June 28, 2019.
2. Singh S, Song R, Johnson AS, McCray E, Hall HI. HIV incidence, prevalence, and undiagnosed infection in U.S. men who have sex with men. Ann Intern Med. 2012;168(10):685-694. doi:10.7326/M17-2082
3. Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection in the United States and Dependent Areas, 2017. CDC website. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. Published November 2018. Accessed June 28, 2019.
4. Dunn K, Lafeuille MH, Jiao X, et al. Risk factors, health care resource utilization, and costs associated with nonadherence to antiretrovirals in Medicaid-Insured Patients with HIV. J Man Care Spec Pharm. 2018;24(10):1040-1051. doi:10.18553/jmcp.2018.17507
5. Rosenblatt L, Buikema AR, Seare J, et al. Economic outcomes of first-line regimen switching among stable patients with HIV. J Am Care Spec Pharm. 2017;23(7):725-734. doi:10.18553/jmcp.2017.16403
6. Gulick RM. Investigational antiretroviral drugs: What is coming down the pipeline. Top Antivir Med. 2018;25(4):127-132.
7. HIV treatment. AIDSinfo website. https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-approved-hiv-medicines. Last reviewed June 24, 2019. Accessed June 28, 2019.
8. Kangethe A, Polson M, Lord TC, Evangelatos T, Oglesby A. Real-world health plan data analysis: key trends in medication adherence and overall costs in patients with HIV. J Mang Care Spec Pharm. 2019;25(1):88-93. doi:10.18553/jmcp.2019.25.1.088
9. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321(22):2203-2213. doi:10.1001/jama.2019.6390
10. Centers for Disease Control and Prevention (CDC). US Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2017 Update: A Clinical Practice Guideline. CDC website. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed June 28, 2019.
11. Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(46):1291-1295. doi:10.15585/mmwr.mm6446a4
12. Sullivan PS, GilerRM, Mouhanna F, et al. Trends in the use of oral emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infection, United States, 2012-2017. Ann Epidemiol. 2018;28(12):833-840. doi:10.1016/j.annepidem.2018.06.009












