Pain Management: How The Opioid Misuse Crisis Impacts Formularies
The United States represents 5.4% of the world’s population; however, it consumes 80% of the world’s opioid supply, according to data from OptumRX. Further, every 16 minutes someone in the United States dies from an opioid related-cause and 4.5 million Americans are estimated to have a substance use disorder related to opioid prescription painkillers.
The opioid misuse crisis also comes with a direct financial burden. The United States spends $504 billion annually on the opioid abuse epidemic—with these costs trending upward based on the 21% increase in drug overdoes deaths reported in 2016. According to the National Center for Health Statistics, death from common prescription opioids, illicit opioids, and a combination of the two, have increased significantly since 2000.
As a result, payers and states have imposed restrictions on the amount of opioids a provider can prescribe to a patient—usually limiting dosage supplies to a single week. However, despite a decrease in per capita prescribing rates between 2012 and 2015, deaths from illicit opioids increased by 134% during this period. This trend may suggest that restricting prescription opioid access without proper patient interventions forces opioid abusers to seek out more dangerous street drug formulations like heroin and fentanyl. Additionally, according to data from OptumRX, 80% of illicit opioid users report that they started out by abusing prescription opioids.
Abuse-Deterrent Opioid Formulations
Having seen a crisis in the making, many manufacturers produced abuse-deterrent opioid formulations—pills with chemical or chemical barriers that make them harder to dissolve, crush, and snort. Further, FDA Commissioner Scott Gottlieb, MD, made it a mission of the FDA to reduce opioid misuse by any means necessary within the Agency. This included encouraging manufacturers to produce more abuse-deterrent formulations. In 2013, the FDA published a guidance for industry on evaluating and labeling formulations of opioids that meet standards of abuse deterrence. To date, there are 10 abuse-deterrent opioid formulations with an FDA label available that meet FDA approval for labeling as abuse-deterrent (Table).
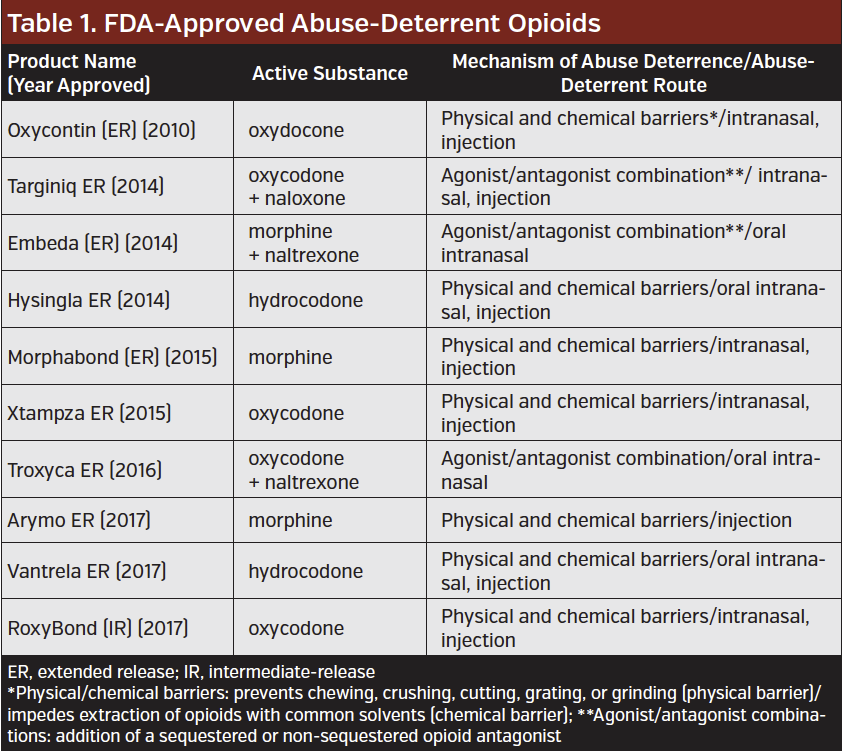
However, few data are available to prove that abuse-deterrent formulation actually deter misuse of the drugs. As a result, the FDA recently launched an initiative to better understand the real-world impact of abuse-deterrent formulations.
Unfortunately, the added layer of abuse protection comes at a cost. Opioids with abuse-deterrent mechanisms are almost double the cost of opioids without the mechanism. ICER reported that the average annual cost of an abuse deterrent formulation prescription (90 mg morphine equivalent dose) was estimated at $4234 compared to $2124 for a nonabuse-deterrent formulation prescription. Additionally, ICER found that it would cost patients and insurers $645 million over 5 years if all opioid medications were made with ADFs.
Despite this, Charles Argoff, MD, president, American Academy of Pain Medicine Foundation, and professor of neurology at the Albany Medical College, advocates for the widespread use of abuse-deterrent formulations.
“Payers and pharmaceutical companies need to harmonize their approach to this so that more people have easier access to these agents so that we can see their true impact and change the direction in their development if the current direction isn’t helping to achieve our outcome of effective prescribing with more safe outcomes,” he told First Report Managed Care.
Formulary Impacts
Despite this burden, recent research published in Medical Care, found that formulary coverage of drugs to treat opioid misuse are still lacking. Haiden A Huskamp, PhD, of the Department of Health Care Policy at Harvard Medical School, and colleagues found that 14% of plans do not cover any formulation of buprenorphine or naloxone—drugs used to treat opioid use disorder. Further, plans were more likely to require prior authorization for drugs to treat opioid misuse than they were for short-acting opioid pain medications (63.6% vs 19.4%).
“Many marketplace plans either do not cover or require prior authorization for coverage of opioid use disorder medications, and these restrictions are often more common for opioid use disorder medications than for short-acting opioid pain medications,” Dr Huskamp and colleagues wrote. “Regulators tasked with enforcement of the Mental Health Parity and Addiction Equity Act, which requires that standards for formulary design for mental health and substance use disorder drugs be comparable to those for other medications, should focus attention on formulary coverage of opioid use disorder medications.”










