Latest Drug Treatment Developments for Type 2 Diabetes in Patients With Cardiovascular Risk
 Cardiovascular disease (CVD) is the leading cause of early death in diabetic patients with at least 68% of patients aged 65 years or older dying from some form of heart disease. In the majority of developed countries, including North America, between 15% to 41% of diabetic patients with a mean age of 50 to 69 years already have CVD. Per the CDC, there are 29.1 million diagnosed and undiagnosed diabetics in the United States, accounting for an associated morbidity and mortality that makes up a substantial portion of our health care expenditures.
Cardiovascular disease (CVD) is the leading cause of early death in diabetic patients with at least 68% of patients aged 65 years or older dying from some form of heart disease. In the majority of developed countries, including North America, between 15% to 41% of diabetic patients with a mean age of 50 to 69 years already have CVD. Per the CDC, there are 29.1 million diagnosed and undiagnosed diabetics in the United States, accounting for an associated morbidity and mortality that makes up a substantial portion of our health care expenditures.
CARDIOVASCULAR RISK REDUCTION
Of those patients diagnosed with diabetes, 90% have type 2 diabetes mellitus. CV risk reduction strategies to date have focused on the management of hyperlipidemia and hypertension. There is also compelling data to support microvascular risk reduction in type 2 diabetes mellitus patients with improved long-term glycemic control. Glycemic control in these studies has been based on changes in HbA1c. However, glycemic control in type 2 diabetes mellitus patients has not been able to demonstrate reduced macrovascular outcomes (CV death, nonfatal myocardial infarction (MI), heart failure, and nonfatal stroke) in clinical trials.
In 2008, given the adverse CV events associated with Avandia (rosiglitazone; GlaxoSmithKline), the risk of CV events in type 2 diabetes mellitus, and the lack of CV outcomes trials (CVOTs) for diabetes medications, the FDA mandated CVOTs for all new antidiabetic therapies. The aim of CVOTs being to demonstrate safety and that new therapy does not result in an unacceptable increase in CV risk. As part of this mandate, the FDA released a document outlining their guidance for industry in evaluating CV risk in new antidiabetic therapies. Per this guidance document, companies are required to enroll patients at high CV risk including those with advanced disease, elderly, and those with renal impairment and gather at least 2 years of CV safety data.
CURRENT CARDIOVASCULAR OUTCOMES TRIALS
Since FDA guidance implementation in 2008, multiple clinical trials have been released to assess CV effects of various new antidiabetic medication classes. The first of which were released for the DPP-4 inhibitor class in 2013 including the SAVOR-TIMI 53 (Onglyza [saxagliptin; AstraZeneca]) and EXAMINE (Nesina [alogliptin; Takeda]) trials followed by TECOS (Januvia [sitagliptin; Merck]) in 2015. Results from these clinical trials were successful in showing a neutral effect of the DPP-4 class on CV events. However, the SAVOR-TIMI 53 trial did demonstrate an unexpected 27% increase in relative risk of hospitalization for heart failure with Onglyza (P = .007).
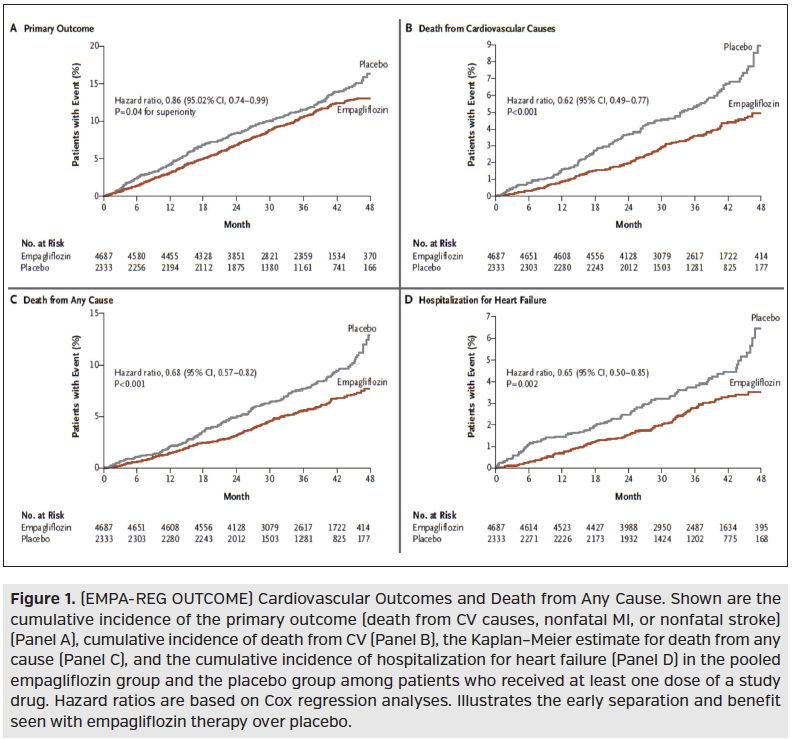
Following the DPP-4 inhibitors, the SGLT-2 inhibitor Jardiance (empagliflozin; Boehringer Ingelheim/Eli Lilly) released its CVOT, EMPA-REG OUTCOME, in 2015. This was a randomized, double-blind, placebo-controlled trial of 7020 patients assessing the effect of Jardiance (10 mg or 25 mg) vs placebo in addition to standard care in type 2 diabetes mellitus patients at high risk for CV events. High CV risk was defined as having one or more of the following: history of recent MI, single or multivessel CAD, unstable angina, history of recent stroke, or occlusive peripheral artery disease. The majority of patients were Caucasian (~72%) male, aged 63 years, with 95% on antihypertensive agents, 89% on anticoagulants/antiplatelets, and 81% on lipid-lowering agents. Over a median observation time of 3 years, the primary outcome of death from CV causes, nonfatal MI, or nonfatal stroke occurred significantly less in the Jardiance treated group demonstrating noninferiority and superiority over placebo. The significance of the primary outcome being primarily driven by the significant reduction in death from CV causes (HR, 0.62; 95% CI, 0.49-0.77; P < .001), with no significant between-group difference for the risk of MI or stroke, Figure 1. Significance was also found for death from any cause (HR, 0.68; 95% CI, 0.57-0.82; P < .001) and reduction of hospitalization for heart failure (HR, 0.65; 95% CI, 0.50-0.85; P = .002).
Continued on next page
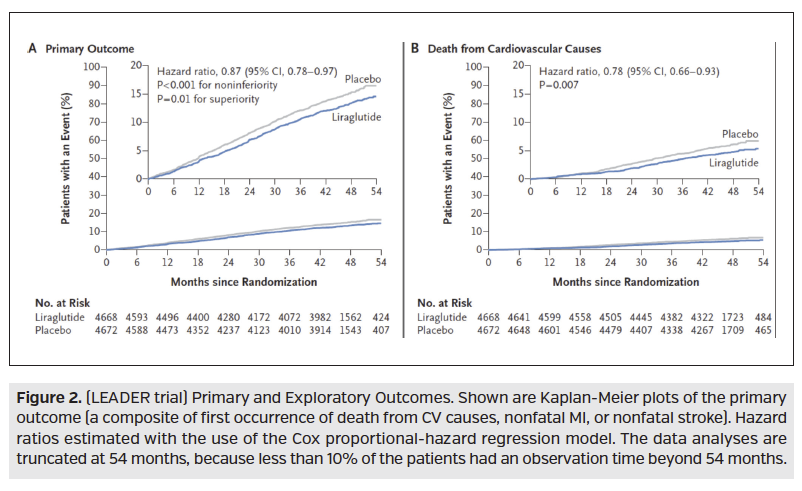
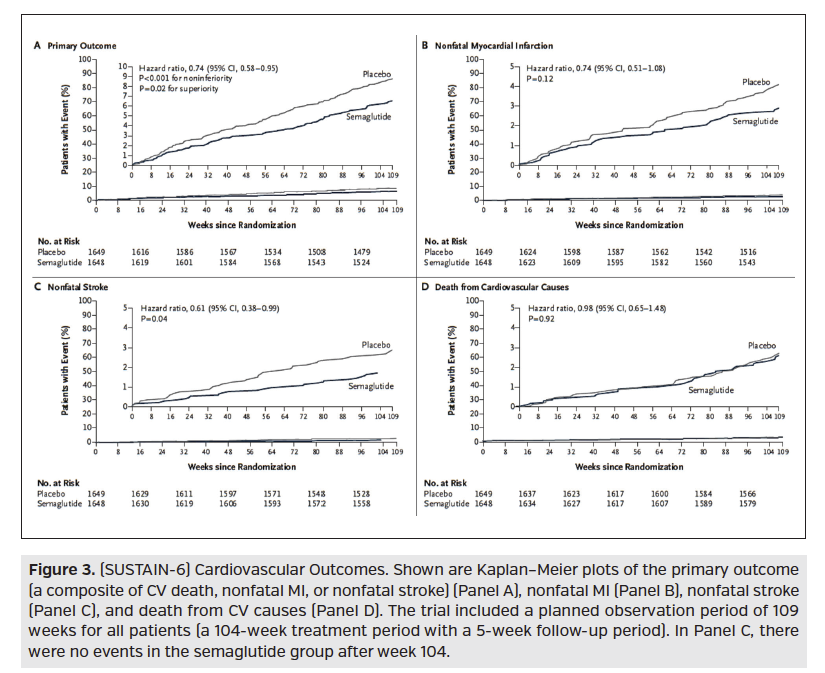
Also recently studied for CV implications, was the GLP-1 receptor agonist medication class. ELIXA was a CVOT studying Adlyxin (lixisenatide; Sanofi), which found no significant difference between treatment and placebo for increase risk or reduction of CV outcomes. In 2016, the LEADER trial, which was a randomized, double-blind, placebo-controlled trial, compared Victoza (liraglutide; Novo Nordisk) 1.8 mg to placebo in 9340 type 2 diabetes mellitus patients with established or high CV disease risk. Over a median follow-up of 3.8 years, the trial demonstrated that the composite outcome (first occurrence of death from CV causes, nonfatal MI, or nonfatal stroke) occurred significantly less in the Victoza treated group over placebo, demonstrating noninferiority and superiority. This statistical significance was primarily driven by a reduction in death from CV causes (HR, 0.78; 95% CI, 0.66-0.93; P = 0.007), Figure 2. Also in 2016, SUSTAIN-6 was a randomized, placebo-controlled trial comparing semaglutide 0.5 mg or 1.0 mg weekly to volume-matched placebo in 3297 high CV risk type 2 diabetes mellitus patients. Here, the primary endpoint (first occurrence of death from CV causes, nonfatal MI, or nonfatal stroke) again proved noninferiority and superiority with semaglutide treatment over placebo. However, unlike the LEADER trial, statistical significance was primarily driven by occurrence of nonfatal stroke (HR, 0.61; 95% CI, 0.38-0.99; P = .04), (Figure 3).
Continued on next page
FUTURE DIRECTIONS
Indeed, FDA guidance has shifted diabetes trials to encourage finding glucose-lowering agents, which are at minimum safe and potentially beneficial for CV events. Based on results found in the EMPA-REG OUTCOME and LEADER CVOTs, the 2017 American Diabetes Association Standards of Care recommend the addition of either Jardiance or Victoza be considered in patients with established CV disease to reduce the risk of mortality. We are currently expecting results from the long awaited CANVAS trial publication mid-2017 to compare Invokana (canagliflozin; Janssen) outcomes against positive results from EMPA-REG OUTCOME and determine if there is a beneficial SGLT-2 class effect. An important point when interpreting a CVOT is that class effect cannot be assumed as illustrated by the differences between thiazolidinediones (Avandia [rosiglitazone maleate; GlaxoSmithKline] and Actos [pioglitazone; Takeda]).
Furthermore, additional studies are needed to assess the CV outcomes with insulin therapy and the upcoming biosimilar emergence, as trials to date have demonstrated a neutral CV impact.
Research is also needed to determine the CV impact of these newer agents in low or moderate CV risk patients as all currently reported CVOTs reflect results for a high CV risk population. And, lastly research with a greater nonwhite population needs to be performed to translate the CVOT findings to races with a higher prevalence of diabetes and heart disease.
IMPLICATIONS FOR MANAGED CARE
As payers look to adopting the CVOT results for improvement in diabetes quality and cost of care management, it is important for organizations to understand: 1. Percentage of their population that has diabetes and high CV risk, and 2. Population race diversity. Per FDA guidance, the CVOTs only include patients with high CV risk (defined earlier in commentary). Knowing that high blood pressure, obesity, and diabetes are the most common conditions that increase the risk of heart disease and stroke, there is a higher prevalence of these conditions with the African American population compared to non-Hispanic white population, treatment responses for heart disease has shown inter-racial disparity, and majority of patients included in CVOTs have been white, it is unknown if the observed CVOT results can be applied to all nonwhite populations. Therefore, adding agents with superiority CVOT outcomes to all patients with diabetes could increase payer's prescription drug costs with unknown medical cost offset secondary to reduced CV events.
Understanding that the identified additional research may not be published in the near future, payers will have to make some decisions on application of published CVOTs for management of their population. One population subset that payers may see an earlier medical cost offset is those with diabetes and congestive heart failure (CHF). The EMPA-REG OUTCOME demonstrated a decline in hospitalization rate for patients with diabetes and CHF early in therapy (Figure 1). To assess the quality of care and cost of care impact of promoting drugs with positive CVOTs, payers should assess their population’s baseline CV event rate and CHF hospitalization rate and reassess the rates for patients started on these drugs biannually or at least annually.










