ADVERTISEMENT
Inhibitory Effects of Short-Chain Fatty Acids and ω-3 Polyunsaturated Fatty Acids on Profibrotic Factors in Dermal Fibroblasts
| Inhibitory Effects of Short-Chain Fatty Acids and ω-3 Polyunsaturated Fatty Acids on Profibrotic Factors in Dermal Fibroblasts | |
| ,a,b ,b ,b ,c ,b ,b ,b ,b ,a ,c b,d | |
aDepartment of Rehabilitation Science; bDivision of Nutrition and Metabolism, Department of Biophysics, Kobe University Graduate School of Health Sciences, Kobe, Japan; cDepartment of Plastic Surgery, Kobe University Graduate School of Medicine, Kobe, Japan; and dFaculty of Clinical Nutrition and Dietetics, Konan Women’s University, Kobe, Japan |
|
Correspondence: nmaeshige@pearl.kobe-u.ac.jp |
|
| This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (no. 23592648). | |
| Keywords: ω-3 polyunsaturated fatty acid, short-chain fatty acid, dermal fibroblast, pro-fibrotic factor, proinflammatory factor | |
Objective: Dermal fibroproliferative disorders impair patients’ quality of life. Although several therapeutic approaches exist for treatment of dermal scars, the development of effective ointments with few adverse effects could improve these therapeutic methods. Short-chain and ω-3 polyunsaturated fatty acids are reported to be immunomodulators with anti-inflammatory properties. Our aim was to evaluate anti-inflammatory and antifibrogenic effects of these fatty acids in human dermal fibroblasts. Methods: Cells were incubated with short-chain fatty acids (butyrate or propionate; 0-16 mM) and/or ω-3 polyunsaturated fatty acids (docosahexaenoic acid or eicosapentaenoic acid; 0-100 μM) for 24 hours to evaluate antifibrogenic effects and for 3 or 48 hours to evaluate anti-inflammatory effects after stimulation with lipopolysaccharide or without stimulation. Expression levels of α-smooth muscle actin, collagen I, collagen III, and IL-6 were evaluated, as were cell proliferation, stress fiber formation, and histone acetylation. Results: In the lipopolysaccharide-unstimulated group, butyrate inhibited mRNA expression of α-smooth muscle actin and collagen III more effectively than propionate and increased histone acetylation. Docosahexaenoic acid inhibited mRNA expression of α-smooth muscle actin and collagen III, whereas eicosapentaenoic acid did not. Combining butyrate with docosahexaenoic acid had stronger effects, downregulating α-smooth muscle actin, collagen I, and collagen III mRNA. As for cell proliferation and stress fiber formation, butyrate acted as a stronger inhibitor than docosahexaenoic acid and the combined administration had stronger effects. In the lipopolysaccharide-stimulated group, butyrate and docosahexaenoic acid attenuated IL-6 mRNA upregulation by lipopolysaccharide. Conclusion: Butyrate and docosahexaenoic acid may be a novel therapeutic approach to treatment of dermal fibroproliferative disorders. |
Dermal fibroproliferative disorders, such as hypertrophic scars, impair the patients’ quality of life owing to cosmetic and functional deficiencies.1 These conditions are caused by aberrant wound healing after any injury or skin incision and are characterized by hyperproliferation of dermal fibroblasts and overproduction of the extracellular matrix, especially type I collagen (collagen I) and collagen III.2 α-Smooth muscle actin (α-SMA) expression and stress fiber formation are observed in the cytoplasm of activated fibroblasts, which undergo intense fibrogenesis, and transforming growth factor β1 (TGF-β1) is reported to be one of the augmenting factors of dermal fibrosis.3 Therefore, regulation of these factors is important for preventing the progression of fibrosis. Furthermore, chronic inflammation activates the aforementioned fibrogenic responses.4 In particular, overexpression of the IL-6 gene in hypertrophic scar fibroblasts has been reported.5 Therefore, regulation of inflammatory responses is needed for scar management. Several therapeutic modalities exist for preventing hypertrophic scar formation, including silicon-based products and radiation therapy, and multiple therapeutic approaches are applied in response to a patient's symptoms.6 Although intraregional treatments using steroids or 5-fluorouracil are known to have promising therapeutic effects,7 the adverse effects of these treatments are considered potentially problematic.
Short-chain fatty acids (SCFAs) are the end products of anaerobic bacterial fermentation of indigestible carbohydrates in the colon.8 Predominantly, butyrate and propionate possess strong physiological activities as histone deacetylase (HDAC) inhibitors.8 We have revealed the inhibitory effects of butyrate and propionate on nuclear factor kappa B (NF-κB) activation in peripheral blood mononuclear cells.9 Recently, the antifibrogenic effect of butyrate in several mesenchymal, rat pancreatic, or hepatic stellate cells was reported,10,11 revealing inhibition of cell growth, collagen III production, and α-SMA expression. However, SCFAs effective at suppression of dermal fibrogenesis are unknown.
Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are primary fatty acids among ω-3 polyunsaturated fatty acids (PUFAs) found in products derived from marine organisms, including fish oil. Higher levels of arachidonic acid, one of the ω-6 PUFAs, have been detected in hypertrophic scars compared with healthy dermis samples.12 Although ω-6 PUFAs possess proinflammatory effects,13 ω-3 PUFAs have anti-inflammatory effects via their lipid mediators.14 Therefore, these fatty acids could be an effective treatment option for dermal fibroproliferative disorders. The antifibrogenic effect of DHA in human peritoneal fibroblasts has been reported, including inhibition of vascular endothelial growth factor and collagen I expression.15 Although both DHA and EPA are purported to possess individual therapeutic activities, the effectiveness of these fatty acids against dermal fibrosis remains unclear.
We hypothesized that SCFAs and ω-3 PUFAs possess inhibitory effects on the expression of profibrotic and proinflammatory factors in dermal fibroblasts. In this study, our objective was to investigate the possible antifibrogenic and anti-inflammatory effects of SCFAs (butyrate and propionate) and ω-3 PUFAs (DHA and EPA) and of their combined administration in human dermal fibroblasts (HDFs). To evaluate the anti-inflammatory effects, we stimulated HDFs with lipopolysaccharide (LPS; derived from Pseudomonas aeruginosa), which is the major pathogenic factor of burn infections.16
MATERIALS AND METHODS
Cell culture
HDFs (CC-2511; Clonetics, San Diego, Calif) were grown at 37°C and 5% CO2 in 100-mm tissue culture dishes (Iwaki, Tokyo, Japan) containing Dulbecco's modified Eagle's medium (DMEM; Dainippon Sumitomo Pharma, Osaka, Japan) supplemented with 10% of fetal bovine serum (FBS) (Nichirei, Tokyo, Japan), penicillin (50 U/mL), and streptomycin (50 μg/mL; MP Biomedicals, Illkirch, France). The cells at passages 5 to 8 were used for experimentation. Because normal fibroblasts increase own α-SMA expression, stress fiber formation, and proliferation when cultured on a plastic substrate in the presence of FBS,3 we seeded HDFs in plastic plates with 10% of FBS to evaluate the suppressive effects of SCFAs and PUFAs on these factors. The cell concentrations were 2.8 × 105/well in 6-well flat-bottom plates (Iwaki) and 8.0 × 103/well in 96-well flat-bottom plates (Iwaki). After 24 hours, fatty acids were added and cells were incubated for additional 24 hours to evaluate the antifibrogenic effects because of the absence of inhibitory effects at 12 hours on mRNA expression of profibrotic factors in our preliminary experiments. Anti-inflammatory effects were evaluated at 3 to 48 hours after administration of LPS and fatty acids. HDFs were subsequently processed for total RNA isolation using the TRIzol Reagent (Invitrogen, Carlsbad, Calif) and for protein isolation using ProPrep (iNtRON, Gyeonggi-do, South Korea). Trypan blue staining was performed to distinguish live cells from dead cells in order to calculate cell viability.
Fatty acid administration
Sodium butyrate (Sigma-Aldrich, St Louis, Mo) and sodium propionate (Sigma-Aldrich) served as SCFAs, whereas sodium DHA (Sigma-Aldrich) and sodium EPA (Sigma-Aldrich) were used as ω-3 PUFAs. To evaluate single-agent administration, butyrate or propionate was applied at a concentration of 0, 1, 4, or 16 mM and DHA or EPA was applied at a concentration of 0, 50, or 100 μM, based on our previous in vitro studies.9,17-21 To evaluate combined agent administration, butyrate at a concentration of 0, 1, 4, or 16 mM was applied in combination with DHA at a concentration of 100 μM.
Proliferation assays
HDFs were seeded in 96-well plates and treated with fatty acids for 24 hours, followed by analysis using the 5-bromo-20-deoxyuridine (BrdU) incorporation assay (Roche, Basel, Switzerland).
Quantitative real-time polymerase chain reaction analysis
RNA concentration and purity were determined by means of absorbance at 260 and 280 nm. First-strand cDNAs were synthesized from the isolated RNAs with the First-Strand cDNA Synthesis System for reverse transcription-polymerase chain reaction (PCR) (Invitrogen). cDNAs were used for subsequent quantitative real-time PCR analysis using the SYBR Premix Ex Taq II (Takara Bio, Otsu, Japan) with appropriate primers (Table 1). The PCR reactions were run on an iCycler IQ (Bio-Rad, Hercules, Calif) for 40 cycles at 95°C for 30 seconds, at an annealing temperature (Table 1) for 30 seconds, and at 72°C for 30 seconds. Post-PCR melting curves were confirmed by the specificity of single-target amplification. All measurements were done in duplicate. All specific quantities were normalized to GAPDH. For each sample, the threshold cycle (Ct) was calculated on the basis of the cycle at which the fluorescence increased above a threshold level. The Ct values were calculated for every sample for the target genes as follows: Ct (target gene) − Ct (internal control gene); GAPDH served as the internal control gene. The relative expression level for 1 target gene (ΔΔCt) was calculated by subtraction of the ΔCt of each control sample from the ΔCt of each experimental sample. Finally, the relative expression value, normalized to an endogenous reference, was calculated as 2−ΔΔCt.
| Table 1. Primers used for real-time polymerase chain reaction | |||
| Annealing, | |||
| Gene | Forward (5′-3′) | Reverse (5′-3′) | °C |
| GAPDH | CATCAAGAAGGTGGTGAAGC | CCTCCCCAGCAAGAATGTCT | 62.5 |
| α-SMA | CGTGGGTGACGAAGCACAG | GGTGGGATGCTCTTCAGGG | 62.5 |
| TGF-β1 | GGGACTATCCACCTGCAAGA | CCTCCTTGGCGTAGTAGTCG | 62.5 |
| Collagen I | GTGCTAAAGGTGCCAATGGT | ACCAGGTTCACCGCTGTTAC | 57.5 |
| Collagen III | TATCGAACACGCAAGGCTGTGAGA | GGCCAACGTCCACACCAAATTCTT | 65.8 |
| IL-6 | AAGCCAGAGCTGTGCAGATGAGTA | TGTCCTGCAGCCACTGGTTC | 60.0 |
Western blotting
A solution of 5 μL of the extracted protein was used to measure protein concentration by Lowry's method (RC DC Protein Assay Kit; Bio-Rad). Western blotting was carried out to determine histone acetylation. In brief, samples containing equal amounts of protein were subjected to electrophoresis in 12% polyacrylamide gels, transferred to polyvinylidene difluoride membranes (GE Healthcare, Buckinghamshire, England), and blocked with 5% non-fat milk. The membranes were incubated with primary antibodies against acetylated histone (1:1000; Cell Signaling Technology Inc, Danvers, Colo) and GAPDH (1:40,000; Sigma-Aldrich) overnight at 4°C, followed by incubation with the appropriate horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature. The blots were then developed using the ECL-plus Western Blotting Detection System (GE Healthcare) and exposed on Hyperfilm (GE Healthcare). Densitometric results were analyzed using the ImageJ software (National Institutes of Health, Bethesda, Md).
Immunofluorescent staining
Stress fibers and nuclear morphology in HDFs were analyzed by immunofluorescent staining. In this experiment, HDFs were seeded on culture cover slips (25-mm diameter; Matsunami Glass Inc, Ltd, Osaka, Japan). HDFs were treated with butyrate at a concentration of 0 or 16 mM in the presence or absence of 100 μM DHA and next were fixed in 4% paraformaldehyde for 30 minutes, followed by immersion in a blocking solution consisting of 1% FBS in Tris-buffered saline (TBS) for 10 minutes at room temperature. After a wash in TBS, F-actin was labeled with phallotoxins (1:40; Invitrogen) for 20 minutes. Finally, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000 dilution; Dojin, Kumamoto, Japan). Immunofluorescent staining patterns were examined under a BX50 fluorescence microscope at ×200 magnification (Olympus, Tokyo, Japan) and recorded with a digital camera (EOS Kiss X4; Canon, Tokyo, Japan).
Statistical analysis
The data are expressed as mean ± SEM. Differences were considered statistically significant at P < .05, as determined by the Tukey-Kramer post hoc test.
RESULTS
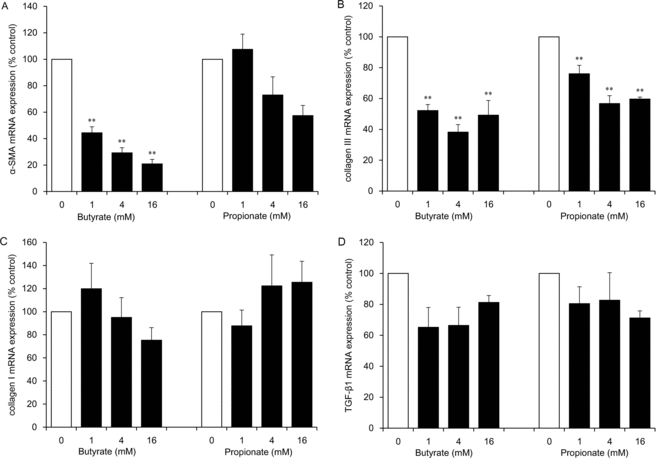
Stronger inhibition of α-SMA and collagen III mRNA expression by butyrate than by propionate in HDFs
Butyrate at concentrations of 1, 4, and 16 mM inhibited α-SMA mRNA expression in a significant and dose-dependent manner (to 44%, 29%, and 21% of the control level, respectively; P < .01) and collagen III mRNA expression (to 52%, 38%, and 49% of the control level, respectively; P < .01; Figs 1a and 1b). Propionate at concentrations of 11, 4, and 16 mM significantly inhibited collagen III mRNA expression (to 76%, 57%, and 60% of the control level, respectively; P < .01), which is a lower degree of inhibition than that observed with butyrate (Fig 1b). Significant differences in collagen I and TGF-β1 mRNA expression levels were not observed (Figs 1c and 1d).
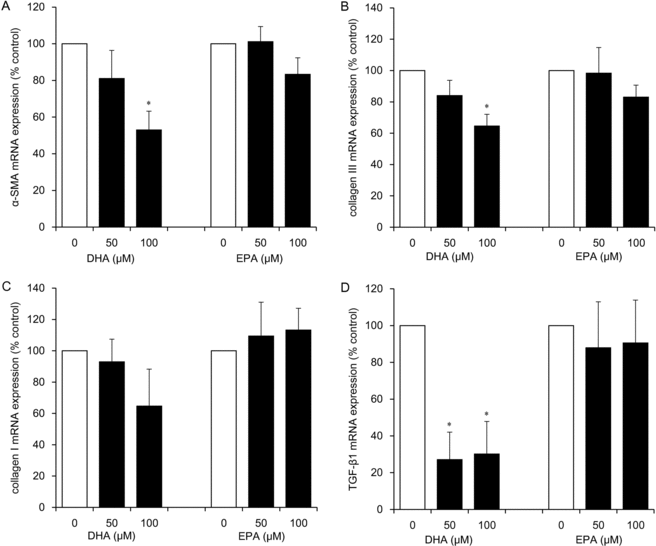
Inhibitory effects of DHA on α-SMA, TGF-β1, and collagen III mRNA expression levels in HDFs
DHA at concentrations of 50 and 100 μM significantly inhibited mRNA expression of TGF-β1 (to 27% and 30% of the control level, respectively; P < .05) and at a concentration of 100 μM downregulated α-SMA (to 53% of the control level; P < .05) and collagen III (to 65% of the control level; P < .05; Figs 2a and 2b–2d). The inhibitory effects of 100 μM DHA on collagen I mRNA expression were not significant (Fig 2c), and no inhibitory effects of EPA were observed (Figs 2a–2d).
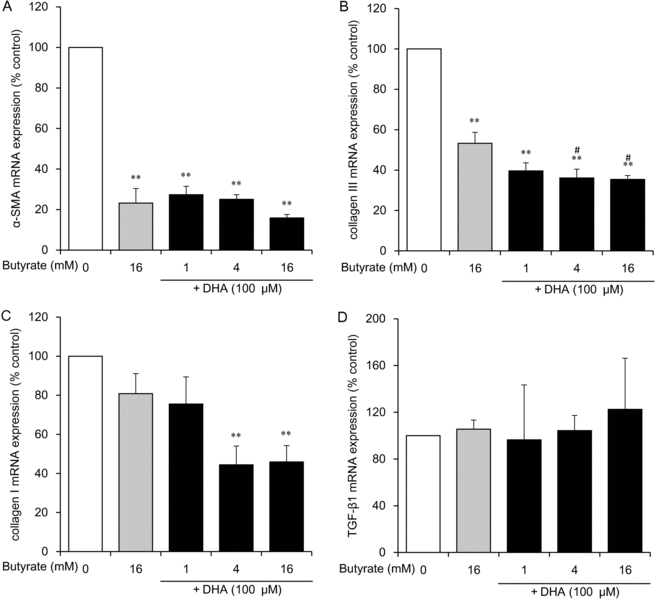
Combined administration of butyrate and DHA exerts strong antifibrogenic effects in HDFs
We expected strong inhibitory effects of the combined administration of butyrate (1-16 mM) and DHA (100 μM). Butyrate at concentrations of 1, 4, and 16 mM combined with 100 μM DHA strongly inhibited mRNA expression of α-SMA (to 27%, 25%, and 16% of the control level, respectively; P < .01), collagen III (to 40%, 36%, and 35% of the control level, respectively; P < .01), and collagen I at concentrations of 4 and 16 mM (to 53% and 46% of the control level, respectively; P < .05; Figs 3a–3c). On the contrary, the combination of butyrate and DHA did not alter mRNA expression of TGF-β1 (Fig 3d).
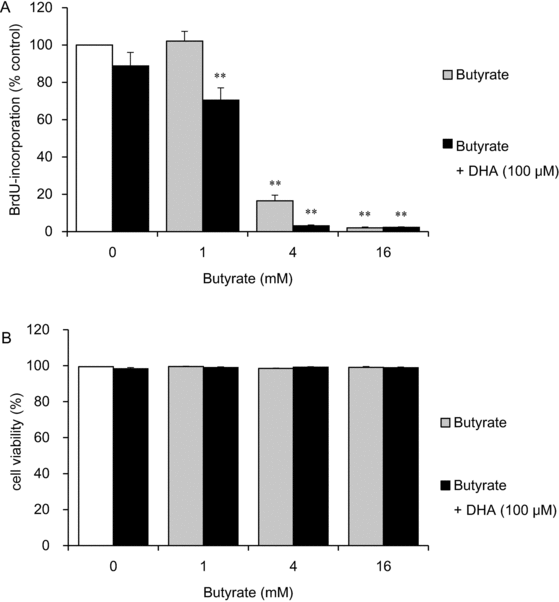
Next, we assessed the effects of butyrate and DHA coadministration on cell proliferation (Fig 4a). Butyrate at concentrations of 4 and 16 mM significantly inhibited cell proliferation (to 17% and 2% of the control level, respectively; P < .01). Butyrate at a concentration of 1, 4, or 16 mM combined with 100 μM DHA significantly inhibited cell proliferation even more than did butyrate treatment alone (to 70%, 3%, and 2% of the control level, respectively; P < .01). Cell counting after trypan blue staining revealed that butyrate and DHA did not decrease cell viability, which remained more than 98% (Fig 4b).
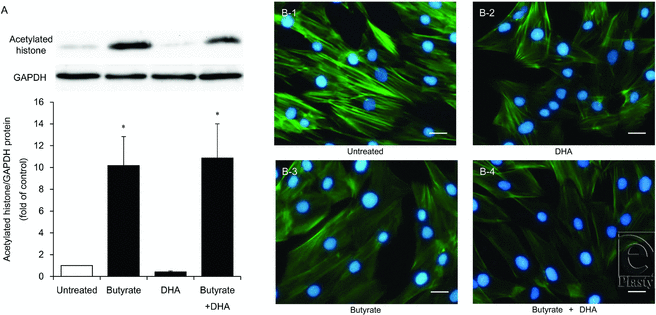
Histone acetylation after butyrate treatment of HDFs
Butyrate at a concentration of 16 mM significantly increased histone acetylation by 10.2-fold (P < .05), but DHA did not. Combined administration of butyrate and DHA increased histone acetylation by 10.9-fold (P < .05) just as administration of butyrate alone did (Fig 5a).
Stress fiber alteration by butyrate and DHA treatment of HDFs
Immunofluorescent staining was carried out to determine the localization of stress fibers (Fig 5b). Double staining for DNA (with DAPI) and F-actin indicated that 16 mM butyrate and 100 μM DHA each decreased stress fiber formation in HDFs, with the effect of butyrate being stronger. Furthermore, butyrate combined with DHA strongly disrupted stress fiber formation in the cytoplasm. To elucidate the effects of these fatty acids on apoptosis, we also evaluated the degree of DNA condensation and fragmentation in HDF nuclei. No increase in DNA condensation or fragmentation was observed after the combined butyrate + DHA treatment for 24 or 48 hours.
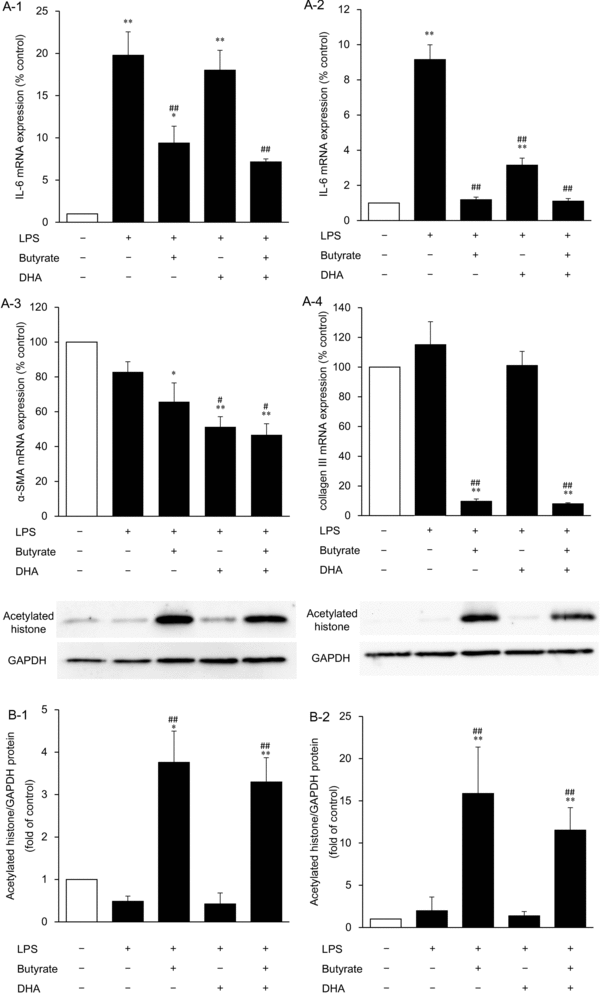
Inhibition of IL-6 and profibrotic factor expression by butyrate and DHA and histone acetylation by butyrate in LPS-stimulated HDFs
To evaluate the anti-inflammatory effects and the relation between anti-inflammatory and antifibrogenic effects, we analyzed IL-6 mRNA expression, which is reported to be upregulated in hypertrophic scars, and α-SMA and collagen III expression levels, which showed major changes under the influence of butyrate and DHA administration in LPS-unstimulated HDFs. In preliminary assays, significant increases in IL-6 mRNA expression were observed at 3, 6, 12, 24, and 48 hours after LPS addition to the medium. Therefore, we evaluated the effects of 16 mM butyrate and 100 μM DHA on IL-6 expression at 3 and 48 hours. Although LPS increased IL-6 expression at 3 and 48 hours by 20.0- and 9.2-fold, respectively (P < .01), administration of butyrate alone and combined administration of butyrate and DHA attenuated this upregulation at 3 and 48 hours (P < .01) and DHA alone did so at 48 hours (P < .01; Figs 6a-1 and 6a-2). As for the profibrotic factors, 16 mM butyrate alone or combined administration of butyrate and 100 μM DHA inhibited α-SMA (to 66% and 47% of the control level, respectively; P < .05) and collagen III (to 10% and 8% that of control level, respectively; P < .01) expression levels; DHA inhibited α-SMA expression (to 51% of the control level; P < .01) at 48 hours in the LPS-stimulated HDFs compared with the control level, whereas LPS did not increase α-SMA and collagen III expression in HDFs (Figs 6a-3 and 6a-4). Finally, we analyzed histone acetylation at 3 and 48 hours after fatty acid administration to evaluate butyrate's action as an HDAC inhibitor in LPS-stimulated HDFs. Administration of butyrate alone and combined administration of butyrate and DHA strongly increased histone acetylation not only at 48 hours (P < .01; Fig 6b-2) but also at 3 hours (P < .05; Fig 6b-1).
DISCUSSION
This study shows the inhibitory effects on the expressions of profibrotic factors and proinflammatory factors by butyrate and DHA in HDFs, both individually and after combined administration. Butyrate had stronger inhibitory effects than propionate on α-SMA and collagen III expression, along with a strong reduction in cell proliferation, stress fiber formation, and LPS-induced IL-6 expression. DHA also inhibited mRNA expression of α-SMA and collagen III and LPS-induced IL-6 mRNA expression. The combined administration of butyrate and DHA augmented the inhibitory effects of butyrate on α-SMA and collagen III mRNA expression, cell proliferation, and stress fiber formation while decreasing the expression of collagen I mRNA. These findings are suggestive of the efficacy of butyrate and DHA as a combined treatment of dermal fibrosis.
In the present study, both butyrate and propionate exerted the inhibitory effects on the profibrotic factor expressions in HDFs, indicating a therapeutic potential of SCFAs against the dermal fibrotic response. The predominance of butyrate in this effect is in agreement with the results of our previous report on NF-κB regulation in peripheral blood mononuclear cells,9 pointing to the potency of physiological action of butyrate. Although TGF-β1 has been shown to augment α-SMA expression,3 butyrate did not alter TGF-β1 expression here. In contrast, butyrate strongly inhibited stress fiber formation in HDFs. Because stress fiber formation upregulates α-SMA expression in fibroblasts,22 the inhibitory effects on profibrotic factor expressions of butyrate are likely to be mediated by stress fiber alteration rather than cytokine regulation. As for anti-inflammatory effects, inhibition of IL-6 mRNA expression by butyrate was observed at 3 hours, as was greater histone acetylation in LPS-stimulated HDFs. This result is consistent with the report of Grabiec et al,23 showing IL-6 mRNA downregulation at 4 hours after administration of trichostatin A, a potent HDAC inhibitor. Therefore, it seems that IL-6 inhibition in the present study is due to the HDAC inhibitory action. LPS stimulation increased IL-6 expression but did not increase expression of profibrotic factors in HDFs. On the contrary, butyrate decreased expression levels of profibrotic factors in the LPS-stimulated group compared with the no-stimulation control. This inhibitory effect on profibrotic factors of butyrate therefore is likely to be independent from IL-6 regulation in this in vitro experiment on monolayer culture. However, this inhibitory action on IL-6 expression may exert therapeutic effects on scar tissue because the high expression of IL-6 in hypertrophic scars has been reported.5
We revealed the inhibitory effects on not only proinflammatory factors but also profibrotic factors by DHA in HDFs. ω-3 PUFAs have been shown to have anti-inflammatory effects.15 Along with these known effects, inhibition of IL-6 expression was observed in the present study. However, the independence of the inhibitory effects on profibrotic factors from that on proinflammatory factors was demonstrated in DHA-treated HDFs and butyrate-treated HDFs. Therefore, it appears that the inhibitory effects on profibrotic factor expressions of DHA are mediated by the mechanisms other than inflammatory factors. After the administration of DHA alone, TGF-β1 mRNA expression was significantly inhibited, as was α-SMA and collagen III mRNA expression. However, the dose dependence of the action of DHA on the α-SMA and collagen III mRNA expression levels was not consistent with that of TGF-β1. Therefore, regulation of TGF-β1 expression does not seem to be the primary mechanism underlying these inhibitory effects. In the present study, the antifibrogenic effect was exerted by DHA rather than EPA. D-series resolvins, protectins, and maresins are lipid mediators derived from DHA but not from EPA.16 Although the roles of these mediators in the DHA-induced inhibitory effects on profibrotic factors can be hypothesized, the direct inhibitory effects of these mediators on fibrogenic responses have not been demonstrated. To elucidate the mechanisms of DHA-induced inhibitory effects on profibrotic factors, detailed experiments examining the metabolism of ω-3 PUFAs and lipid mediators are required.
The cooperative inhibitory effect of combined butyrate and DHA administration on profibrotic factor expressions was revealed in the present study. DHA administration not only enhanced the antifibrogenic effects of butyrate but also inhibited collagen I mRNA expression by approximately 50%, indicating a more potent antifibrogenic effects than single fatty acid administration. Recently, the proapoptotic effect of combined butyrate and DHA administration on colonocytes was demonstrated.24 In the present study, proapoptotic morphological changes, such as DNA fragmentation or condensation, were not observed in the fatty acid–treated fibroblasts. Therefore, further studies are necessary to reveal this effect or its absence by means of primary culture of fibroblasts derived from pathological tissue such as keloids.
In the present study, we revealed the inhibitory effects on profibrotic factor expressions using the cultured human fibroblasts. Meanwhile, the mechanism of scaring in human is very complicated.1 Therefore, we cannot determine the antifibrogenic effects of these fatty acids in the present study. To develop the antifibrogenic therapy using these fatty acids, future clinical studies are required.
In summary, we report the inhibitory effects on profibrotic and proinflammatory factor expression by butyrate and DHA on HDFs, both individually and in combination. These findings can contribute to the development of novel therapies for dermal fibrosis. Further experiments are necessary to elucidate the mechanism behind these effects.
ACKNOWLEDGMENTS
The authors thank Drs Shunsuke Sakakibara and Shingo Kamoshida for their assistance with immunofluorescence staining.
1. Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids—a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35(2):171-81. |
2. Syed F, Ahmadi E, Iqbal SA, et al. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol. 2011;164(1):83-96. |
3. Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;35:349-63. |
4. Deitch EA, Wheelahan TM, Rose MP, et al. Hypertrophic burn scars: analysis of variables. J Trauma. 1983;23(10):895-8. |
5. Xue H, McCauley RL, Zhang W, et al. Altered interleukin-6 expression in fibroblasts from hypertrophic burn scars. J Burn Care Rehabil. 2000;21(2):142-6. |
6. Kim S, Choi TH, Liu W, et al. Update on scar management: guidelines for treating Asian patients. Plast Reconstr Surg. 2013;132(6):1580-9. |
7. Ledon JA, Savas J, Franca K, et al. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39(12):1745-57. |
8. Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time?. Nutr Clin Pract. 2006;21(4):351-66. |
9. Usami M, Kishimoto K, Ohata A, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28(5):321-8. |
10. Bülow R, Fitzner B, Sparmann G, et al. Antifibrogenic effects of histone deacetylase inhibitors on pancreatic stellate cells. Biochem Pharmacol. 2007;74(12):1747-57. |
11. Niki T, Rombouts K, De Bleser P, et al. A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology. 1999;29(3):858-67. |
12. Nomura T, Terashi H, Omori M, et al. Lipid analysis of normal dermis and hypertrophic scars. Wound Repair Regen. 2007;15(6):833-7. |
13. Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51(3):232-7. |
14. Lee HN, Surh YJ. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochem Pharmacol. 2012;84(10):1340-50. |
15. Victory R, Saed GM, Diamond MP. Antiadhesion effects of docosahexaenoic acid on normal human peritoneal and adhesion fibroblasts. Fertil Steril. 2007;88(6):1657-62. |
16. Nasser S, Mabrouk A, Maher A. Colonization of burn wounds in Ain Shams University Burn Unit. Burns. 2003;29(3):229-33. |
17. Usami M, Komurasaki T, Hanada A, et al. Effect of gamma-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition. 2003;19(2):150-6. |
18. Usami M, Muraki K, Iwamoto M, et al. Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells. Clin Nutr. 2001;20(4):351-9. |
19. Ohata A, Usami M, Miyoshi M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition. 2005;21(7/8):838-47. |
20. Miyoshi M, Usami M, Ohata A. Short-chain fatty acids and trichostatin A alter tight junction permeability in human umbilical vein endothelial cells. Nutrition. 2008;24(11/12):1189-98. |
21. Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition. 2010;26(6):653-61. |
22. Sandbo N, Lau A, Kach J, et al. Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β. Am J Physiol Lung Cell Mol Physiol. 2011;301(5):L656-66. |
23. Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann Rheum Dis. 2012;71(3):424-31. |
24. Kolar S, Barhoumi R, Jones CK, et al. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca2+ loading and apoptosis in colonocytes. Cancer. 2011;117(23):5294-303. |
| JOURNAL INFORMATION | ARTICLE INFORMATION |
| Journal ID: ePlasty | Volume: 19 |
| ISSN: 1937-5719 | E-location ID: e4 |
| Publisher: Open Science Company, LLC | Published: March 1, 2019 |