Women and Atrial Fibrillation: Persistent Higher Risk of Morbidity and Mortality
Atrial fibrillation (AF) is the most common sustained arrhythmia, causing affected patients a significant decrease in quality of life, morbidity, and mortality. Women with AF have a higher burden of symptoms and risks compared to men. The cost of financial burden in the management of AF patients is also high, ranging from approximately $16 to $26 billion in the United States.1 This cost is due to hospitalizations, outpatient care, medical and non-medical treatment, as well as complications from treatments, especially major bleeding.2
Sex-specific differences with AF can be found in the epidemiology, pathophysiology, presentation, outcomes, and response to therapy. Although women have a lower incidence of AF than men, due to the increased longevity of women and higher number of women compared to men, AF affects as many women as men. Similar to coronary artery disease, the average age of women with AF is about 7 years older than men.3
Mortality Risk
A meta-analysis of 30 studies published in 2016 with over 4.3 million participants found that women had a 12% increased risk of death compared to men (95% CI, 1.07 to 1.17). Compared to those without AF, the relative risk (RR) in mortality was higher in women compared with men: RR is 1.69 (95% CI, 1.50 to 1.90) and 1.47 (95% CI, 1.32 to 1.65), respectively.4
Stroke Prevention and Risk
Women are undertreated compared to men for stroke prevention, and have a higher risk of stroke even when treated with warfarin. In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study, women were found to spend less time in therapeutic range compared to men. Additionally, even when women had comparable time in therapeutic range on warfarin, they still had a higher risk of systemic thromboembolism.5 However, novel oral anticoagulants (NOACs) had better stroke prevention outcomes for women with AF relative to men. The meta-analysis of warfarin compared to NOACs showed that there were no sex differences in outcomes for stroke, thromboembolism, and major bleeding with the use of NOACs.6 A meta-analysis of the four available NOACs showed no clinically significant sex differences with their use; all were equally efficacious in decreasing stroke and thromboemboli.7
Recent studies continue to show that women with AF have worse outcomes in terms of strokes and mortality compared to men.3 The National Cardiovascular Data Registry’s (NCDR®) PINNACLE Registry revealed that compared to men, women are less likely to receive anticoagulation. The reasons for this difference in the prescription of stroke-preventing medications are unclear, and undoubtedly contribute to the higher adverse events and mortality in women.1 No sex differences in anticoagulant therapy were found in the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) study: women (60.8%) and men (60.9%).8
Updates to the American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for AF, published in 2019, changed the Class I recommendation of starting oral anticoagulation in women with non-valvular AF (defined as those without moderate-to-severe mitral stenosis or a mechanical heart valve). The guidelines continued to recommend the use of the CHA2DS2-VASc score to calculate the stroke risk of patients with AF. CHA2DS2-VASc assigns 1 point to six risk factors (congestive heart failure [systolic and diastolic]), hypertension, diabetes, vascular disease, age ≥65 years, and female sex), and 2 points to a history of stroke or transient ischemic attack (TIA) and age ≥75 years, for a maximum of 9 points.9 In women whose CHA2DS2-VASc score is 3 or greater, there is a Class IA recommendation to treat with oral anticoagulants including warfarin, apixaban, dabigatran, edoxaban, or rivaroxaban regardless of the type of AF pattern (paroxysmal, persistent, or permanent). There is a Class IIB recommendation for women with a CHA2DS2-VASc score of 2.10 This modification of the recommendation from treating women with a CHA2DS2-VASc score of ≥2 with an OAC was based on a study that showed that women with 2 or more non-sex based risk factors had a higher risk of stroke or thromboembolism than men.11 However, women with no other risk factors other than female sex had a low risk for stroke or thromboembolism, and did not warrant anticoagulant or antithrombotic therapy. These recommendations are summarized in Table 1.
In high-risk patients, female sex was associated with a twofold increased risk of severe disabling or fatal ischemic stroke in consecutive AF patients with stroke according to the AHA’s Get With The Guidelines®-Stroke database, even after adjustment for possible confounders.12 A population-based study from Quebec found that the 16% higher risk of stroke in women with AF was no longer seen when the men and women were matched by baseline risk factors at entry.13
Left atrial appendage endocardial occlusion devices
Left atrial appendage (LAA) endocardial occlusion devices have been approved for AF patients who are unable to use oral anticoagulants because of bleeding or difficult anticoagulation management. These devices include the WATCHMAN device (Boston Scientific), AMPLATZER Cardiac Plug and AMPLATZER Amulet LAA occluder (Abbott), Coherex WaveCrest LAA Occlusion System (Biosense Webster, Inc., a Johnson & Johnson company), and LARIAT device (AtriCure). An initial study showed that compared to men, women were found to have more incidence of device-related thrombus (75% vs. 34%, P=.094) using the WATCHMAN,14,15 but more recently, no sex difference in device-related thrombus was found.15 A study of the AMPLATZER Cardiac Plug found that female sex (OR: 4.22; P=.027) and cigarette smoking (OR: 5.79, P=.017) were independent predictors of device-related thrombus in univariate and multivariable logistic regression models.16
Presentation
Women with AF were more symptomatic than men. The Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF registry) found that women had more functional impairment, more limitation in their daily activities, and worse quality of life than men; these findings remained true even after accounting for AF therapies received.17
Rate and Rhythm Control
In the late 1990s, studies evaluating rate versus rhythm control of AF showed rate control to be non-inferior to rhythm control.18,19 In the AFFIRM trial, the risk of death did not differ by sex between rate control and rhythm control strategies.5,18 The Rate Control versus Electrical Cardioversion (RACE) trial19 found that women treated with rhythm control had a higher incidence of cardiovascular death, heart failure, thromboembolic complications, adverse effects of antiarrhythmic drugs, and pacemaker implantation compared with women treated with rate control.
There were no sex differences in the use of beta-blocker therapy (72.5% and 70.0%), but women were more likely to be treated with digoxin (25.0% vs 19.8%) than men (P=.0056).20 This is worrisome, particularly since two studies found an association with increased risk of breast cancer in women with AF treated with cardiac glycosides.21,22 Women with AF who were enrolled in the Women’s Health Initiative (n=93,676) from 1994-1998 and followed for 15 years had a 5.7% incidence of invasive breast cancer.21 Accounting for confounders, a 19% excess risk of invasive breast cancer was associated with cardiac glycoside use. The second study was a meta-analysis of 29 studies that showed an association of cardiac glycoside use with estrogen-receptor-positive breast cancer (RR = 1.330, 95% CI: 1.247-1.419) and increased all-cause death (HR = 1.35, 95% CI: 1.248-1.46).22
Antiarrhythmic drug use in women compared to men with AF was associated with increased mortality (95% CI: 1.5 to 6.3; P=.002) and adverse cardiovascular outcomes such as heart failure, thromboembolism, bleeding, need for pacemaker, and severe adverse effects from the antiarrhythmic drugs in the RACE trial.19
Catheter ablation for AF was not an available option for rhythm control when these studies were conducted. Once catheter ablation therapy became more widely available, studies revealed that women are referred less often for catheter ablation as rhythm control therapy for AF. Procedural complications for ablations are higher in women than men, mostly due to bleeding.23 Although the Catheter Ablation vs Antiarrhythmic Drug Therapy for AF (CABANA) trial did not show a significant difference in the primary endpoint of cardiovascular mortality, the recurrence of AF was significantly better with patients who underwent catheter ablation.24
Summary
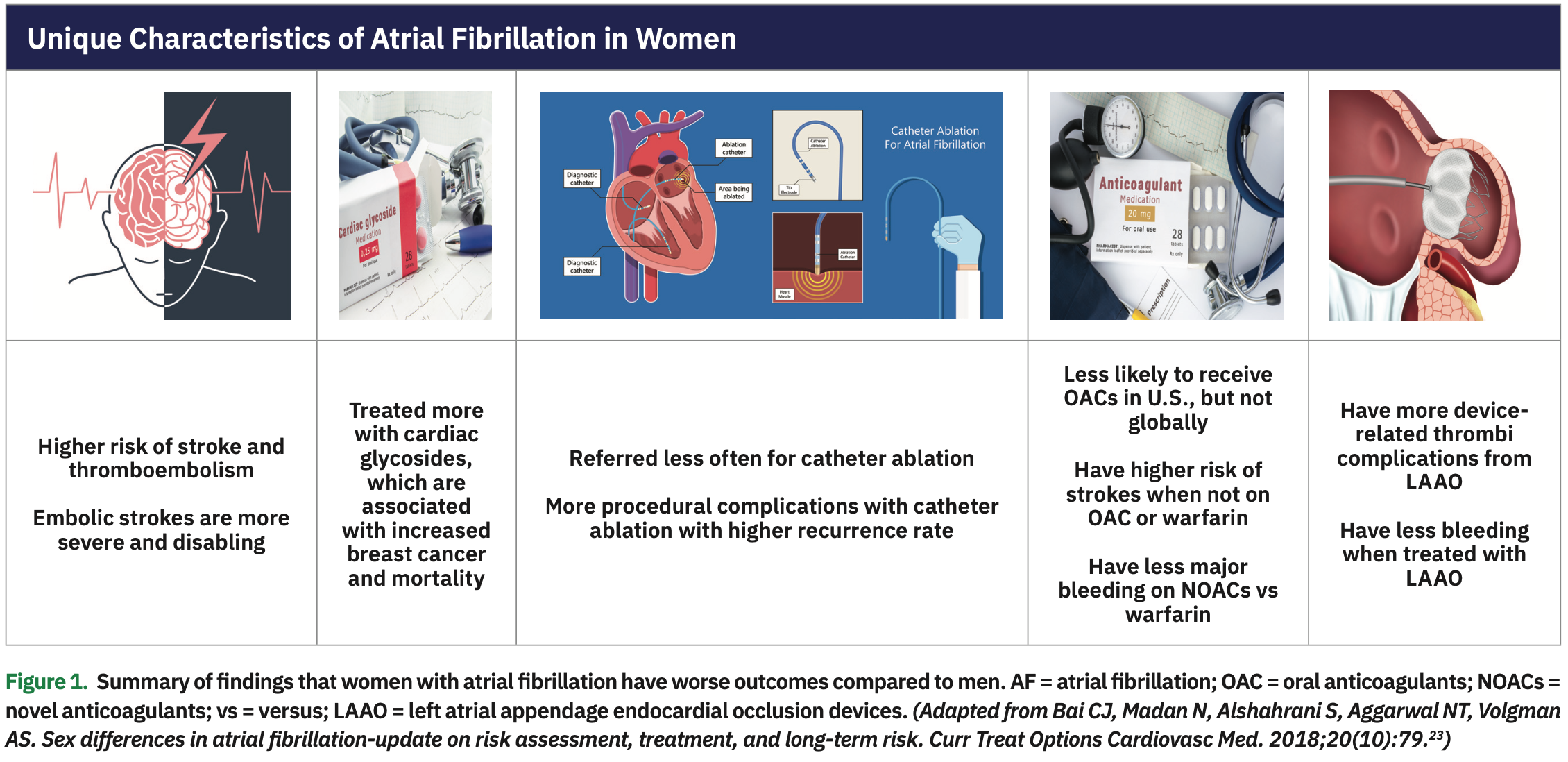
Compared to men, women with AF continue to have worse major adverse cardiovascular outcomes including mortality as well as stroke risk and severity. In addition, women with AF have worse quality of life and treatment complications; these findings are summarized in Figure 1. Women are more often treated with cardiac glycosides compared to men, which is associated with a higher risk of breast cancer and mortality. Women are also referred less often for catheter ablation for rhythm control and have more complications with procedures such as catheter ablation and left atrial appendage endocardial occlusion devices. Table 2 outlines possible solutions to decrease these risks, which include: (1) adherence to stroke prevention guidelines; (2) improved monitoring to detect AF sooner in women who complain of palpitations, shortness of breath, and fatigue; (3) earlier referral for catheter ablation as a first line of treatment for rhythm control; (4) shared decision making of use of oral anticoagulants with preference for NOACs; (5) improved education of risk and benefits of stroke prevention drugs and procedures; (6) avoidance of unnecessary use of digoxin; (7) careful monitoring of electrolytes, QT interval, and side effects; (8) consideration of smaller-size catheters for women to reduce bleeding complications; and (9) assessment of bleeding risk if the patient is on medications that can increase bleeding risk such as aspirin, non-steroidal anti-inflammatory drugs, and fish oils. Continued efforts to decrease morbidity and mortality are essential to improve outcomes in both men and women with AF.
Disclosures: Dr. Volgman reports she is a consultant for the American Heart Association, speaker for Aptus Health, and has stock in Apple, Inc.
- Thompson LE, Maddox TM, Lei L, et al. Sex differences in the use of oral anticoagulants for atrial fibrillation: a report from the National Cardiovascular Data Registry (NCDR®) PINNACLE Registry. J Am Heart Assoc. 2017;6(7).
- Ribeiro AL, Otto CM. Heartbeat: the worldwide burden of atrial fibrillation. Heart. 2018;104(24):1987-1988.
- Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195-203.
- Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013.
- Sullivan RM, Zhang J, Zamba G, Lip GY, Olshansky B. Relation of gender-specific risk of ischemic stroke in patients with atrial fibrillation to differences in warfarin anticoagulation control (from AFFIRM). Am J Cardiol. 2012;110(12):1799-1802.
- Pancholy SB, Sharma PS, Pancholy DS, Patel TM, Callans DJ, Marchlinski FE. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol. 2014;113(3):485-490.
- Moseley A, Doukky R, Williams KA, Jaffer AK, Volgman AS. Indirect comparison of novel oral anticoagulants in women with nonvalvular atrial fibrillation. J Womens Health. 2017;26(3):214-221.
- Lip GY, Rushton-Smith SK, Goldhaber SZ, et al. Does sex affect anticoagulant use for stroke prevention in nonvalvular atrial fibrillation? The prospective global anticoagulant registry in the FIELD-Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8(2 Suppl 1):S12-S20.
- Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10(3):258-266.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132.
- Nielsen PB, Skjøth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137(8):832-840.
- Martin RC, Burgin WS, Schabath MB, et al. Gender-specific differences for risk of disability and death in atrial fibrillation-related stroke. Am J Cardiol. 2017;119(2):256-261.
- Renoux C, Coulombe J, Suissa S. Revisiting sex differences in outcomes in non-valvular atrial fibrillation: a population-based cohort study. Eur Heart J. 2017;38(19):1473-1479.
- Kaneko H, Neuss M, Weissenborn J, Butter C. Predictors of thrombus formation after percutaneous left atrial appendage closure using the WATCHMAN device. Heart Vessels. 2017;32(9):1137-1143.
- Dukkipati SR, Kar S, Holmes DR, et al. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. 2018;138(9):874-885.
- Saw J, Tzikas A, Shakir S, et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the Amplatzer cardiac plug. JACC Cardiovasc Interv. 2017;10(4):391-399.
- Piccini JP, Simon DN, Steinberg BA, et al. Differences in clinical and functional outcomes of atrial fibrillation in women and men: two-year results from the ORBIT-AF Registry. JAMA Cardiol. 2016;1(3):282-291.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833.
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834-1840.
- Lip GY, Laroche C, Boriani G, et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. 2015;17(1):24-31.
- Wassertheil-Smoller S, McGinn AP, Martin L, Rodriguez BL, Stefanick ML, Perez M. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol. 2017;185(5):372-384.
- Osman MH, Farrag E, Selim M, Osman MS, Hasanine A, Selim A. Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS One. 2017;12(6):e0178611.
- Bai CJ, Madan N, Alshahrani S, Aggarwal NT, Volgman AS. Sex differences in atrial fibrillation-update on risk assessment, treatment, and long-term risk. Curr Treat Options Cardiovasc Med. 2018;20(10):79.
- Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274.