Three-Dimensional Printing in Cardiac Electrophysiology: Current Applications and Future Directions
Introduction
With the advancements in therapeutic options offered in today’s cardiac electrophysiology (EP) practice, as well as the increased complexity of patients with congenital and acquired heart disease requiring invasive procedures to treat heart rhythm disorders, there is an increased need for cardiac electrophysiologists to better understand the complexity of their patients’ unique cardiac anatomy. Thus, pre-procedural planning has become essential for a “personalized approach” to patient care in the EP lab. Three-dimensional (3D) printing of cardiac models offers a new dimension of understanding from a visual, tactile, and conceptual perspective. Not only does 3D printing facilitate procedural planning by improving the understanding of a patient’s unique anatomy, which assists in equipment selection and choice of surgical or invasive techniques that are better suited for each individual case, but it also allows for comprehensive procedural “rehearsal” of both established and experimental techniques, potentially reducing procedural times and improving patient outcomes. As the clinical problems of today’s patients are driving higher complexity of procedures to be performed, the training of current and future electrophysiologists must evolve to include cardiac and translational anatomy as an essential component of their curriculum. Three-dimensional printing of normal and abnormal anatomical specimens offers unique opportunities for academic teaching, procedural simulation, and research of new techniques and equipment. In this manuscript, we review the concept of 3D printing and present some of the current uses of 3D printing in clinical, teaching, and research settings within our institution. Finally, we discuss the current limitations and potential applications for this technology in the future.
Workflow for Three-Dimensional Printing
Diagnostic cardiac imaging modalities such as computer tomography (CT) and magnetic resonance imaging (MRI) are stored as universally accepted Digital Imaging and Communications in Medicine (DICOM) files. Using commercially available image processing software, DICOM files undergo a process called segmentation where the anatomical regions of interest (ROI) of a particular data set are selected using a reconstructed model from the 2D CT and MRI slices, often with the use of intravenous contrast agents to help delineate the endoluminal contours of the cardiac chambers and blood vessels (for example, the left atrial and pulmonary vein anatomy in atrial fibrillation ablation cases, or the aortic cusps and coronary arteries for left-sided outflow tract cases).1 These segmented or “target-tissue” images in DICOM format then need to be converted to a compatible file format, such as STL (Standard Tessellation Language or STereoLithography file), to be used for 3D printing. Other common file formats include VRML (Virtual Reality Modeling Language) and AMF (Additive Manufacturing File). Next, the converted file undergoes a process called fixing, which eliminates small imperfections or surface overlaps in the 3D rendered model that occur when converting DICOM to STL formats and are due to artifacts occurring during the original image acquisition. An additional step called design allows for the customization of the 3D model to serve a specific purpose (for example, creating a cutaway view to expose an intracardiac cardiac structure). These steps render a virtual 3D print model, which is essentially a virtual copy of the 3D model to be printed. The 3D virtual model can be used as a PDF file, and be assessed for accuracy of the segmentation and volume rendering prior to printing (thus, serving a quality control role); it can also be used for pre-procedural evaluation using 3D navigational tools that are customizable (such as different projections, cutaway views, transparency, coloring, measurements, etc.). Finally, the ready to print STL file is sent to a 3D printer for the creation of the final 3D printed model. Depending on budget and clinical needs, there are many options for commercially available 3D printers, most of them suitable for medical 3D printer needs. Some examples include stereolithography (which creates models using layers of polymer, rendering rigid 3D models), selective laser sintering (SLS) (which uses laser as a source to sinter powdered resins), PolyJet technology (which allows for dual material printout with soft and hard resin components), binder jetting (inkjet printing into a powder bed of raw material, allowing for multiple color printing) and Fused Deposition Modeling (which essentially uses layers or melted thermoplastic that hardens after extrusion from the nozzle, also known as plastic jet printing).2 In our laboratory, we mainly use stereolithography and PolyJet technology printers for models used in simulation training and pre-procedural rehearsals, and binder jetting printers for heart models that require coloring and highly detailed anatomical reconstruction to be used in cardiac anatomy lectures and research.
Current Uses of 3D Printing in the EP Laboratory
In the cardiac electrophysiology laboratory at our institution, we have implemented the use of 3D printing for over 2 years. Our areas of focus are anatomy lectures and courses, clinical research, and procedural simulation. We collaborate closely with the Brody 3D printing laboratory at the University of Maryland School of Medicine, a non-profit research laboratory working on the applications of cardiac 3D printing in clinical practice and research. We are part of a multidisciplinary team that includes radiologists, adult congenital and pediatric cardiologists, and cardiothoracic surgeons.
Cardiac Anatomy
Knowledge of cardiac anatomy in EP is vital for understanding the association between structural anomalies (congenital and acquired) and their potential arrhythmic complications, either related to the course of the disease or to the effect of medical, surgical, or interventional procedures. From a procedural point of view, it is essential to understand the anatomical relationships between different cardiac and vascular structures pertaining to vascular access and the risks associated with device implants and catheter ablations. Access to cadavers and heart specimens is expensive, time constrained, and limited to local availability; therefore, access to gross anatomy samples for easy consultation is impractical in everyday practice. Although consultation of cardiac anatomy atlases and handbooks is available, the 2D nature of these documents makes for a limited understanding of the spatial relationship of the relevant anatomical structures. A 3D printing lab/workstation offers the advantage of availability, low cost, and customization of models to specific needs. One has the possibility to select both normal and rare anatomical specimens (for example, hypertrophic cardiomyopathy, L-TGA, single ventricle) for teaching and training purposes. Most importantly, these models can be used on multiple occasions and can be reprinted as needed. In our institution, we have developed a cardiac anatomy curriculum combining didactic lectures with virtual 3D modeling and hands-on printed heart model sessions (Figure 1). We have a collection of 3D models of different pathologies, and our goal is to eventually have a complete cardiac anatomy atlas of normal and abnormal 3D heart models as well as virtual files for academic and research use. Additionally, web-based platforms allow for medical file sharing of printable medical files, thus expanding access to an unlimited number of interesting cardiac specimens to a worldwide audience.3
Clinical Research and Procedural Simulation
We are currently focusing our 3D printing efforts on two important clinical areas: pre-procedural planning and rehearsal for left-sided cardiac interventions, and fellows’ procedural training and simulation using cardiac 3D printed models in the EP lab environment.
For left-sided interventions (such as left atrial appendage occlusion procedures), we use a personalized medicine approach including a pre-procedural cardiac CT scan followed by segmentation and 3D virtual model rendering of the left atrial (LA) and left atrial appendage (LAA) anatomy. Evaluation of a virtual 3D model (Figure 2A) allows for measurements of the LAA ostial dimensions essential for selecting the appropriate LAA occluder device size. The relationship between the LAA and the left-sided veins, the size of the LAA ridge, and the angle between the interatrial septum and the long axis of the LAA, are important for sheath selection and for anticipating possible manipulations required during device delivery. Finally, the 3D printed heart model allows for hands-on procedural rehearsal, giving the operator tactile feedback and an opportunity to test which combination of sheath, device, and movements are required to successfully complete the procedure (Figure 2B). Our experience with this methodology is that it facilitates the selection of the correct materials for the case, particularly occluder device sizing (which tends to be underestimated by the transesophageal echo measurements), but also identifies potential anatomical variants such as extreme LAA orientations and small LAA landing zones, where adequate sheath selection as well as pre-rehearsed sheath manipulations and device deployment provide the operator with “muscle memory” and can help them overcome unique anatomical challenges during the actual procedure.
Clinical simulators in cardiac electrophysiology are increasingly being used in fellowship training and for physicians adopting new procedures. However, these simulators are expensive, and their availability is oftentimes limited to company-sponsored events or courses. Moreover, tactile feedback and the sensation of a “real-life” EP laboratory environment are often lacking. Three-dimensional printing presents the opportunity for procedural rehearsal and simulation in a real-life EP environment using patient- and “chamber of interest”-specific models to help physicians improve their procedural skills. In our EP laboratory, we have developed a simple yet effective procedural simulation and training workflow for our fellows-in-training:
Step 1: A pre-procedural CT from our database of cases is selected. The DICOM file is segmented and a 3D virtual model is created.
Step 2: The virtual 3D model is fixed and designed. A 3D PDF file is obtained, and a 3D model is printed using a semi-translucent hard resin (Figure 3A).
Step 3: The fellow evaluates the 3D PDF virtual file and has a hands-on session with the 3D printed model to better understand the relevant cardiac anatomy for the case (Figures 3B and 3C).
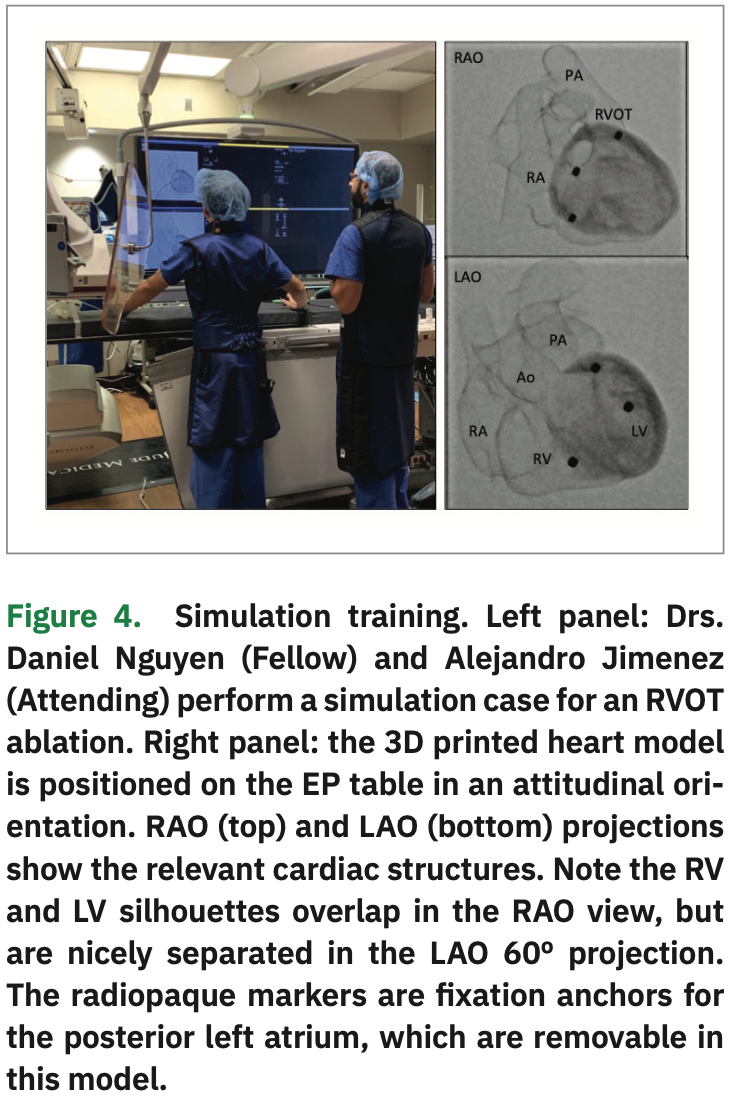
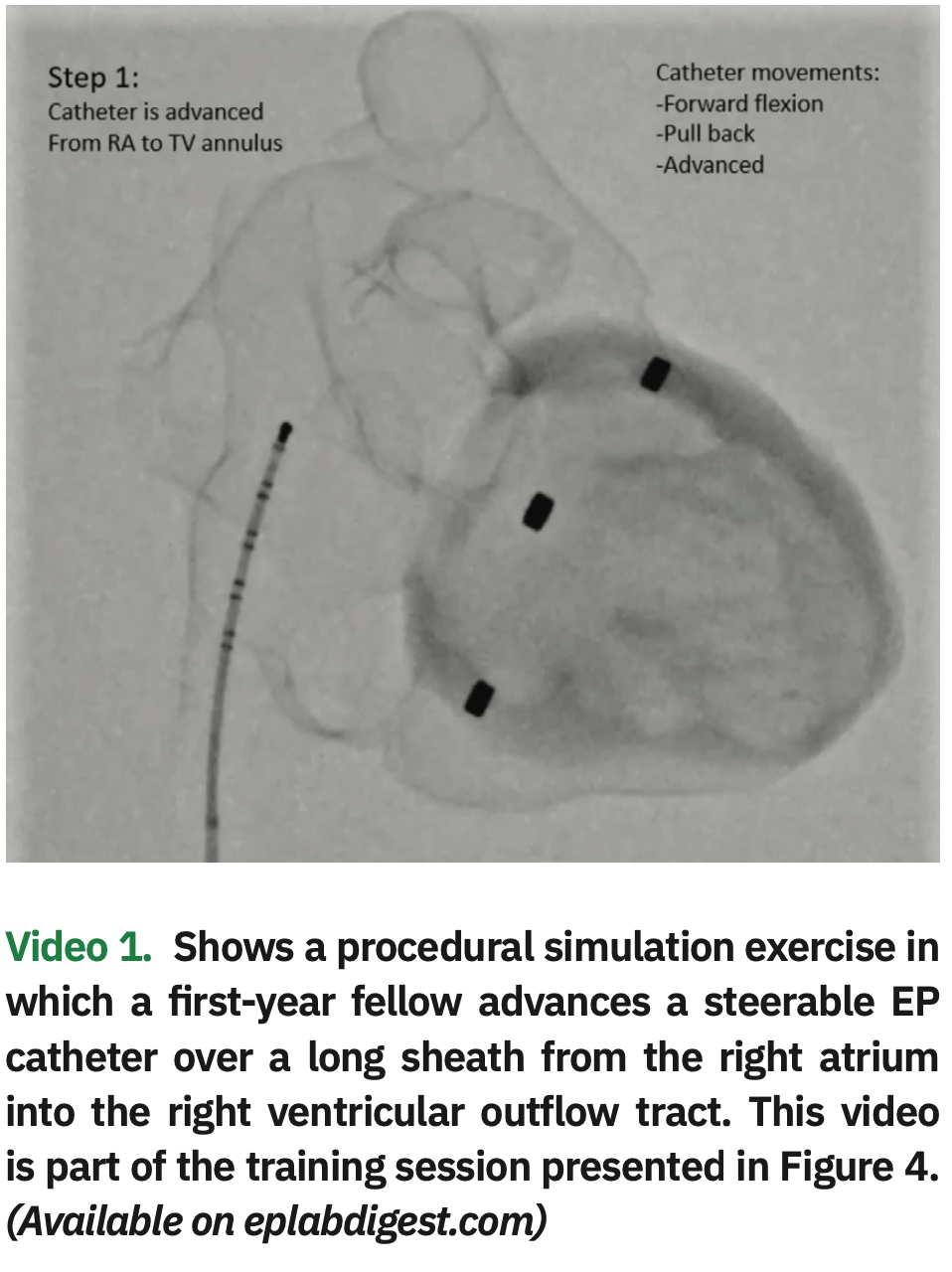
Step 4: The fellow performs a mock procedure using the 3D cardiac model (1:1 scale) placed on the EP procedural table and positioned in an attitudinal orientation, using the same equipment he or she would normally use for an actual case: fluoroscopy, x-ray protection equipment, guidewires, long sheaths, and catheters. An attending physician helps the fellow during the simulation, providing feedback on catheter and sheath movements needed to reach the different anatomical structures (Figure 4 and Video 1, also available at https://bit.ly/2FNSi7o).
This procedural simulation workflow is inexpensive and safe. The cost of a hard resin model using an STL printer is approximately $10 per model. A complete four-chamber cardiac model takes about 10 hours for printing, and 2 hours for curating and finishing. The model can then be used on multiple simulations. The upfront cost of the printer is under $2000. We use expired or previously used and resterilized catheters, guidewires, and sheaths for the simulation cases. All simulation cases are done in accordance with the ALARA principle for radiation safety using minimal radiation exposure (2 frames per seconds fluoro, no cine, collimation, and lead aprons and shielding). Our early experience with these mock procedures using 3D printed models has been well received by our fellows. The 3D models are well visualized and give the appearance of a real heart on fluoroscopy. Although the model material is different to human tissue because the model is anatomically accurate, it gives the fellows an opportunity to develop muscle memory as well as tactile feedback of the motions and rotations required to manipulate the catheters and sheaths inside the heart. It also provides them with a sense of “familiarity” when an actual case is performed.
Limitations
Cardiac 3D printing requires collaboration between radiologists, cardiologists, and technicians. The segmentation, virtual 3D modeling, and 3D printing can be time and labor intensive. Depending on the quality and specifications of the printers utilized, some printed models require 10 or more hours for completion. Once the models are printed, post-processing of the printed model (curating) is necessary to make it clinically useful. Thus, availability and easy access to 3D cardiac models that can be used for clinical practice, research, and training requires financial, and more importantly, human capital resource allocation for a smooth workflow in the 3D printing lab. Resins with flexible and “tissue-like” characteristics are more expensive and only available for more expensive and high-end printers. A learning curve is required to improve the 3D printing workflow of image processing and 3D printing.
With regards to pre-procedural simulation before cardiac interventions and its impact on patient and procedural outcomes, the data are lacking and only limited to anecdotal case reports and small case series.4,5 The added benefit of procedural training using 3D models for fellows-in-training is unknown, and a quantitative assessment of its impact on the learning curve of a given individual would be very difficult to measure, as procedural endpoints are influenced by many other variables. Qualitative data based on post-simulation experience questionnaires are encouraging, but prospective large data collection across different simulation experiences (device implants, ablations, and structural interventions) and different levels of fellows are required to better understand its true impact.
Future Directions
With technical advances and accessible costs for virtual reality (VR) and augmented reality (AR) applications and equipment, the integration of 3D printing with VR and AR simulators is both feasible and logical.6 Both technologies should be viewed as complementary rather than exclusive. The components of the ideal simulator include a 3D model of the arterial and venous vasculature for vascular access simulation, and a central chest unit of the 3D printed heart model of a specific patient (interchangeable) with electronic connections for simulating atrial and ventricular electrograms. A virtual 3D file is uploaded in an AR or VR visualization environment (using goggles or 3D screen), which includes fluoroscopic, 3D mapping images, and intracardiac electrograms. Finally, the model has pressure/contact sensors that are used to provide both tactile and contact force feedback to the operator by vibratory input into the catheter handle and/or sheath. Bioprinting using 3D-printed scaffolds to regenerate human tissue is an exciting avenue for regeneration of diseased cardiac tissue (myocytes, conduction tissue, heart valves).7 In the field of medical device development, the use of 3D printed models of human-specific anatomy made of tissue-like materials can help bypass animal testing in the developing phase of new catheters, leads, and innovative devices. Telemedicine and remote consultation services can be enhanced by sharing of printable files across centers anywhere in the world, where a consulting expert (using his or her own hands) using AR/VR tools and local 3D printing facilities can analyze a specific cardiac model of a patient and provide feedback regarding the feasibility and technique required to perform a specific intervention. Finally, training institutions can develop cardiac anatomy labs based on 3D printed models of normal and abnormal anatomical specimens, with opportunities for teaching and clinical research.
Acknowledgments. We would like to thank Jeffrey Hirsch, MD for sharing his knowledge and expertise with image segmentation, virtual modeling, and 3D printing. We would also like to thank Armand Campos, RN for his invaluable help during simulation cases.
Video 1 is also available at this link: https://www.eplabdigest.com/multimedia/3d-printing-cardiac-electrophysiology-procedural-simulation-exercise
Disclosures: The authors have no conflicts of interest to report regarding the content herein.
Listen to our podcast with Dr. Restrepo in the link below!
- Jimenez A, Dickfeld T. Computer tomography in cardiac electrophysiology. In: Zipes D. Cardiac Electrophysiology: From Cell to Bedside, Seventh Edition. Philadelphia, PA: Elsevier, 2017.
- Bartel T, Rivard A, Jimenez A, Mestres C, Muller S. Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur Heart J. 2018;39(15):1246-1254.
- NIH 3D Print Exchange. U.S. Department of Health and Human Services — National Institutes of Health. Available at https://3dprint.nih.gov/. Accessed December 3, 2019.
- Rossi L, Penela D, Doni L, et al. Development of simulation combining a physical heart model and three-dimensional system for electrophysiology training. Pacing Clin Electrophysiol. 2018;41(11):1461-1466.
- Seslar SP, Patton KK. Initial experience with a novel electrophysiology simulator. Pacing Clin Electrophysiol. 2018;41(2):197-202.
- Jang J, Tschabrunn CM, Barkagan M, et al. Three-dimensional holographic visualization of high-resolution myocardial scar on HoloLens. PLoS One. 2018;13(10):e0205188.
- Ong CS, Fukunishi T, Zhang H, et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017;7(1):4566.