ADVERTISEMENT
A Streamlined Approach to Catheter Ablation for Atrial Fibrillation
Introduction
Atrial fibrillation (AF) is the world’s most common arrhythmia,1 with future incidences and associated costs on healthcare services expected to continue rising.2 Given its known cardiovascular and cerebrovascular-associated morbidity and mortality,3 catheter-based ablation (CBA) and specifically, pulmonary vein isolation (PVI), have become relevant “curative” treatment strategies.4,5 Technological advances, procedural metrics, and patient outcomes continue to improve to the point where PVI is now sometimes considered a routine CBA procedure. Therefore, the onus is on healthcare systems to improve patient outcomes while simultaneously minimizing inpatient stay and resource utilization. In this review, we will explore the various logistic, heuristic, clinical, and technological factors that, when aligned with appropriate protocols, have resulted in the desirable confluence of high success rates, low complication rates, and minimal inpatient stay at our hospital.
Preassessment
Liverpool Heart and Chest Hospital provides tertiary level cardiac services to a population of 2.8 million in northwest England. We offer PVI to patients with paroxysmal AF (PAF) if they are intolerant to, non-responsive to, or unkeen to be on lifelong drug therapy. PVI in persistent AF (persAF, class IIA) is reserved for patients with ongoing symptoms despite rate control medications, or evidence of left ventricular systolic dysfunction (LVSD).6 At our institute, nurse-led preassessment is conducted on all patients referred for PVI.7 Here, diagnosis is confirmed, relevant blood tests are performed, peri-procedural anticoagulation strategies are addressed, and potential day case patients are identified.
Imaging
As left atrial (LA) size is known to predict procedural success rates,8 transthoracic echocardiography (TTE) is performed on all patients prior to PVI. Although no specific maximum cutoff for LA size is recommended,9 if the LA is found to be greater than 50 mm on short axis views or 60 ml by volume, we will often adopt a more judicious approach to offering CBA due to lower reported success rates.10 Use of pre-procedural cardiac computed tomography (CCT) or cardiac magnetic resonance imaging (CMR) is employed on a case-specific basis when additional imaging is felt to be warranted.
Anticoagulation
Large variability exists in stroke-prevention strategies for patients with AF. The emergence of non-vitamin K oral anticoagulants (NOACs)11-14 has revolutionized stroke prevention in patients with non-valvular atrial fibrillation (NVAF), who are now routinely prescribed these medications as first-line therapy. Indeed, at the time of listing for PVI, our patients on vitamin K antagonists (VKA) are offered a switch to a NOAC. Our default “rule of thumb” is to anticoagulate without interruption as much as clinically feasible. Prolonged catheter placement in the arterial circulation as well as LA endothelial damage resulting from ablation can create a highly prothrombotic milieu, leaving patients temporarily at much higher risk for stroke than can be predicted based on their CHA2DS2-VASc score. For patients on VKA, our policy is one of uninterrupted treatment15 with an INR range of 2.0-3.5 on the day considered acceptable.16 For NOACs, we recommend uninterrupted treatment, with the last dose given the previous evening for rivaroxaban and edoxaban, and the same morning for dabigatran and apixaban. The NOAC is then given at the usual dose on the same evening after the procedure.17 Intra-procedural unfractionated heparin is administered to a target activated clotting time (ACT) >300 s, in sequential boluses with an initial dose of 100 IU/kg for patients on warfarin and 130 IU/kg for patients on NOACs, as NOACs tend to associate with greater intra-procedural ACT lability and blunted ACT response.18 Finally, we administer protamine prior to sheath removal to facilitate earlier hemostasis without the need for closure devices or sutures. For patients not anticoagulated prior to ablation, we routinely prescribe a NOAC for 2 months post procedure.
Preparation
Conscious sedation versus general anesthesia (GA)
Given the greater potential for patient discomfort during radiofrequency ablation (RFA), we generally perform these cases using full GA, whereas cryoballoon ablation (cryo-PVI) cases are offered conscious sedation (CS). The use of GA for RFA has an advantage of improved catheter stability and contact force (CF), known to be associated with improved success rates.19 Some centers use deep sedation, in which the cardiologist administers fentanyl and propofol under the supervision of the anesthetist, obviating the need for powerful neuromuscular blocking agents that would otherwise prolong patient recovery.20 A study by Martin et al21 compared use of CS vs GA in 292 consecutive patients undergoing RFA for persAF. They observed a higher freedom from reintervention at 18 months in those who underwent GA vs CS (70.8% vs 57.3%; P=.044), amounting to a cost savings of £178 per patient. All of this taken together suggests that GA for RFA at index procedure, while more expensive and time-consuming in the short term, may be the most cost-efficient way of ensuring a lasting and successful outcome. For cryo-PVI, it appears CS gives similar freedom from AF at 30 days with shorter procedural times and no significant increase in adverse events when compared with GA.22 With careful planning and focused patient recovery, we have developed a safe practice of discharging uncomplicated GA and CS PVIs on the same day.7
Vascular access
A standard mandate at our facility is the use of ultrasound (US)-guidance for all vascular access (Figure 1). In 2014, we demonstrated a large reduction in inadvertent arterial punctures (6.1% vs 13%; P=.04) and BARC (Bleeding Academic Research Consortium) 2+ bleeding (requiring treatment by a healthcare professional, prolonged hospitalization, or increased level of care, 10.5% vs 19.7%; P=.02) in patients who had US-guided venous puncture versus those who did not.23 One should not underestimate the morbidity surrounding vascular complications, as even so-called “minor” complications can culminate in repeat hospital admissions, leading to an unnecessary burden on healthcare resources. We have shown that US-guidance can reduce even “minor” complications such as groin pain (27.1% vs 42.8%; P=.006), use of analgesic medications (9.7% vs 24.6%; P=.001), and prolonged bruising (20.5% vs 40.4%; P=.001). Since 2013, there have been zero reported arteriovenous fistulae or pseudoaneurysms in over 6000 procedures carried out at our institution. We highly recommend US for all vascular access, a simple practice which can result in enormous clinical and pecuniary benefits.
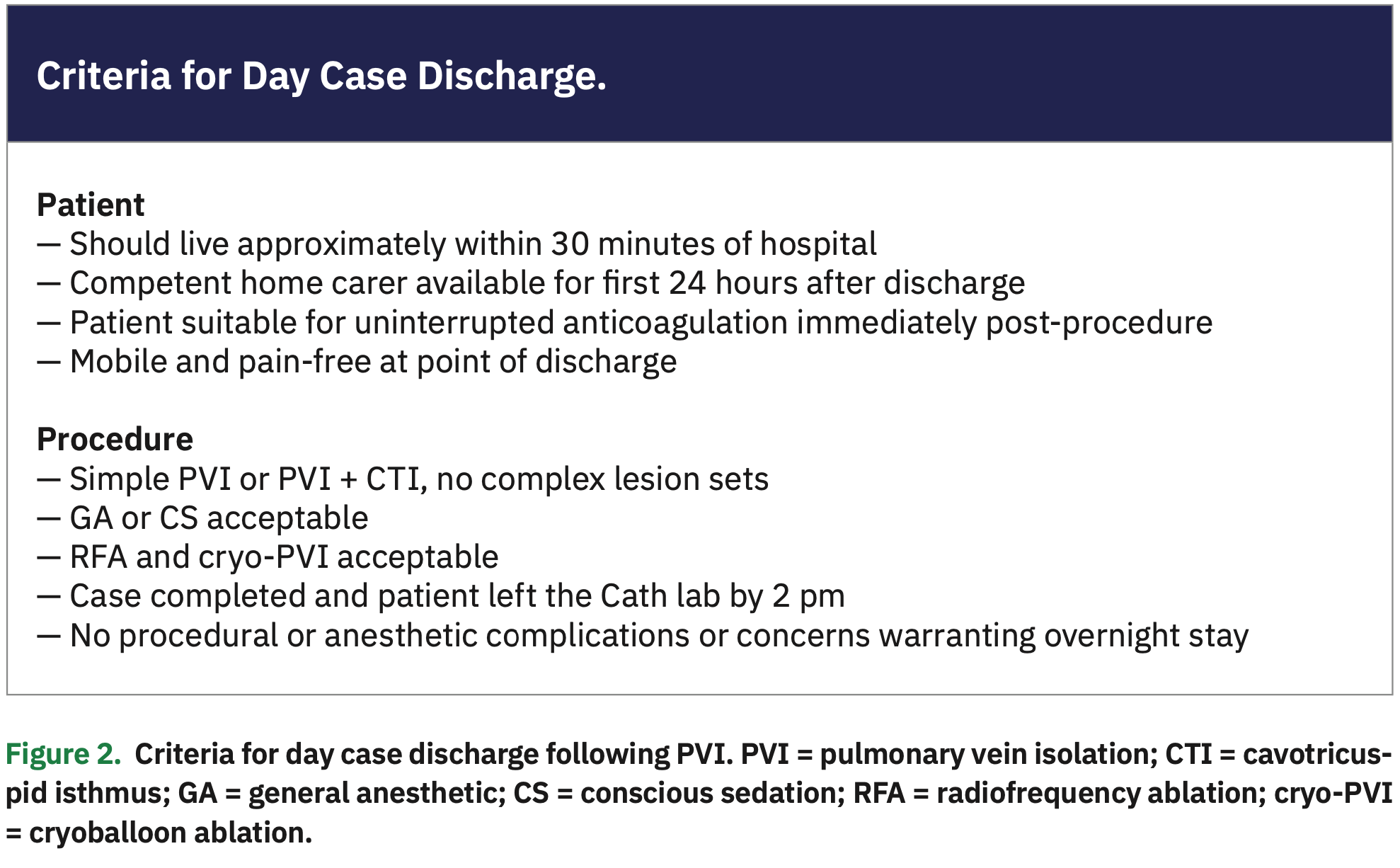
Day case procedures
At our institute, patients are admitted at two time points: 08:00 and 11:00, depending on whether they are listed for the morning or afternoon lists. Our default approach now is for patients on a morning list to be offered same-day discharge. From 2014-2017, 169 (20.8%) of 811 PVI patients on a morning list were discharged on the same day.7 Factors more likely to be associated with same-day discharge included use of conscious sedation (CS) vs general anesthetic (GA) (P<.001), redo vs de novo PVIs (P<.01), absence of additional linear ablation lines after standard PVI wide area circumferential ablation (WACA; P=.004), and absence of the need for intra-procedural electrical cardioversion (P=.003) (Figure 2). Notably, same-day discharge did not confer any additional risk: only 3 (2.1%) day case discharges presented again within 1 week with minor complications (such as pericarditis and vomiting), and all were successfully and conservatively managed. The study compared favorably with other studies looking at day case PVIs, with a comparatively lower incidence of peri-procedural complications.24,25 Our findings suggest that a substantial number of patients electively admitted for PVI can safely be done as day cases.
Procedure
Cryo-PVI vs RFA
Choosing the appropriate CBA modality for PVI can be challenging, and depends on multiple factors including patient anatomy, patient preference concerning sedation, redo vs de novo procedures, and availability of anesthetic cover. Intuitively, one would assume that point-by-point bespoke PVI with RFA would afford a more durable result than a generalized circumferential ostial freeze with cryo-PVI. However, the landmark FIRE AND ICE trial26 showed non-inferior primary outcomes in those undergoing cryo-PVI vs RFA (34.6% vs 35.9%; P=.0004 for non-inferiority), and reduced need for repeat ablations (11.8% vs 17.6%; P=.03) in 762 patients over an 18-month follow-up period. Since then, the second-generation cryoballoon has continued to demonstrate comparable or greater long-term freedom from recurrence of PAF,27,28 with significantly lower peri-procedural complications. It’s important to stress that patients in the FIRE AND ICE trial were excluded if they had significant LA enlargement (>50 mm) and LVSD (EF <35%). Although no head-to-head data exist for cryo-PVI vs RFA for larger LA and LVSD, our practice is to offer cryo-PVI in the first instance to all PAF patients with confirmed structurally normal hearts; subsequent ablations (if required) are performed using RFA. RFA is used at first ablation if there is severe LA enlargement (>50 mm), or if more extensive lesion sets are to be delivered (such as posterior wall isolation or cavotricuspid isthmus ablation). If the patient preference is for GA, we use RFA.
Specific ablation modality targets
How does the electrophysiologist know they have achieved a durable PVI? For RFA, research has refined specific empiric intra-procedural targets with notable success. Initially, there was CF.29 Later, more integrated approaches using additional markers of stability evolved, which most recently includes ablation index (AI): a composite index incorporating CF, time, and power in a weighted formula.30,31 We demonstrated that higher AI values were required (no late reconnection demonstrated at values >480) at the anterior and roof of the LA, and lower values (no reconnection at values >370) at the posterior, inferior, and carinal LA.30 Using higher targets (at our institution, 550 for the anterior LA and roof, and 400 for the posterior and inferior LA) reliably translates to durable PVI,32 although emerging evidence would suggest even more modest targets can be considered.33 Another means of ensuring first-pass PVI is the use of intertag distance (ITD) (VISITAG, Biosense Webster, Inc., a Johnson & Johnson company). ITDs <6 mm have been shown to translate to a fully reinforced WACA line with no breakthrough.34 Evidence for transmurality is supplemented by the use of a live impedance drop graph, a helpful surrogate for CF,35 with a targeted drop of 10-15Ω corresponding to CF of 7-19 g.40 Finally, we use power of 50W for all lesions. Recent published data have shown a dramatic reduction in ablation times (<10 s per lesion at 50W, compared with 30 s per lesion at 35W) with excellent acute, mid-term, and long-term success rates and no associated increase in major complications.36,37 Simultaneous use of all of these targets has translated to a streamlined, reproducible metric set for the operator to confidently achieve first-pass PVI, with impressive mid- to long-term freedom from AF.38
For cryo-PVI, our operators use an ablation strategy that roughly aligns with the protocol from the Cryo-DOSING study39: if PVI is achieved within 60 seconds, the application is continued for 3 minutes; if PVI is achieved between 60-90 seconds, the application is continued for a total of 4 minutes. If PVI has not been achieved after 90 seconds, if PV signals are not visible in real time, or the temperature has not reached -40 degrees after 60 seconds, the application is aborted and the balloon is repositioned. We aim to have a single effective freeze for each PV rather than delivering additional bonus freezes on an empirical basis. Diaphragmatic pacing during right-sided PV freezes is mandatory with diaphragmatic stimulation confirmed on electromyography. In the Cryo-DOSING study,39 the cryo-dosing arm was found to have comparable acute and 1-year success rates versus a conventional cryo-PVI technique (99.6% vs 99.2% immediate PVI and 82.5% vs 78.3% 1-year freedom from atrial arrhythmias, respectively; P=.14) with no significant difference in complications, and a dramatic reduction in total freeze time (16 ± 5 minutes vs 40 ± 14 minutes; P<.01). This algorithm could likely be universally adopted as a means of intensifying procedural efficiency at no extra risk to the patient.
Esophageal protection
By far the most serious complication of PVI, with a very high mortality rate, is atrial esophageal fistula (AOF). It may develop weeks or even months after PVI, and is largely preventable by employing measures to avoid esophageal thermal injury. More cases have been described following RFA than cryo-PVI,40 although it can occur in either. Thus, mandatory intraprocedural esophageal temperature monitoring is used at our facility during RFA of the posterior LA. Ablation is suspended if local esophageal temperature rises above 39ºC until core temperature is restored, although studies suggest temperatures up to 41ºC are allowable.42 Given that over 80% of patients show some endoscopically visible esophageal abnormality following posterior wall ablation,41 it is our practice to give all patients post-RFA PVI a prescription for a high-dose proton pump inhibitor or histamine-2 (H2) antagonist for at least 1 month.
Conclusion
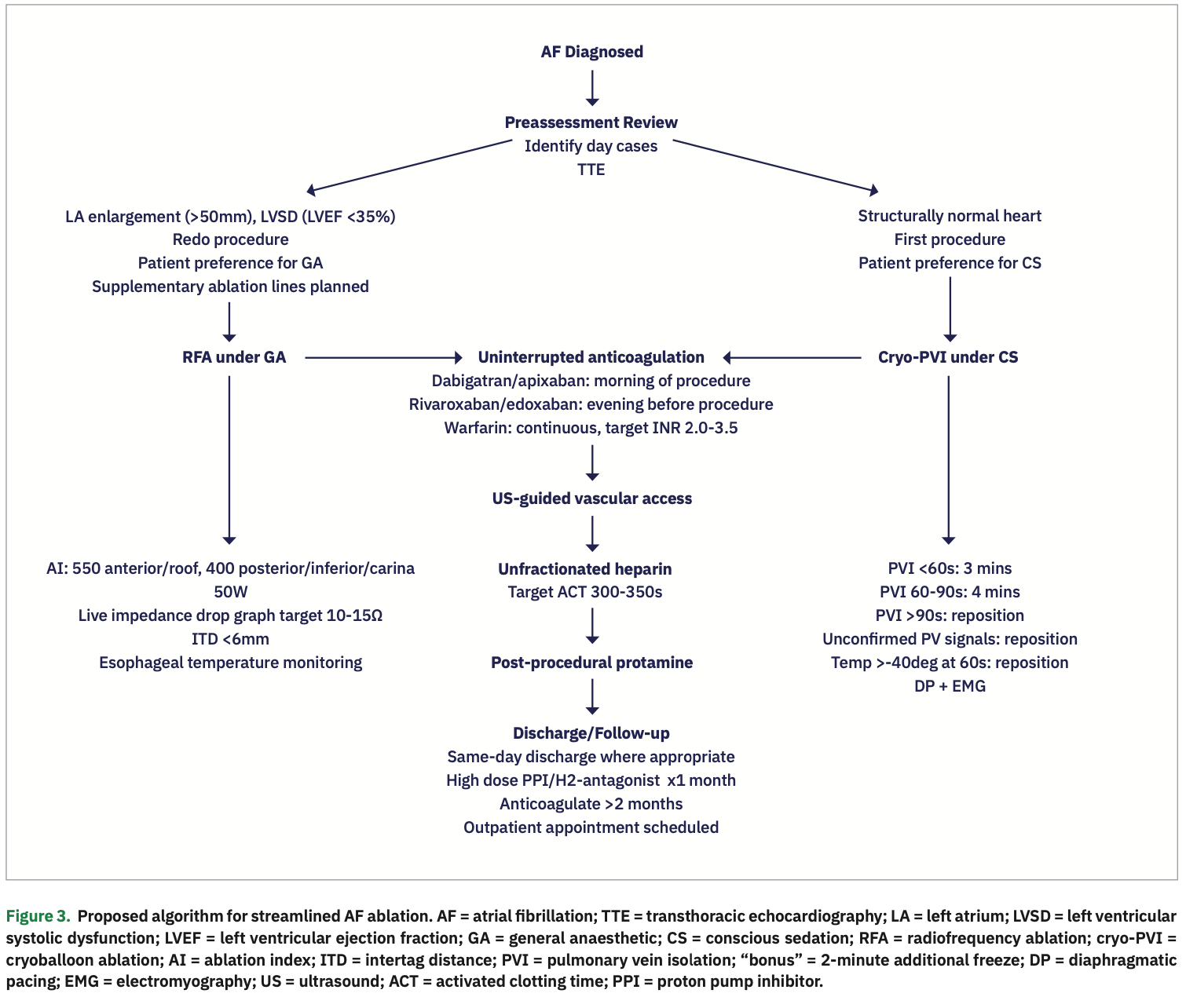
Significant improvements in the efficacy and safety of catheter ablation for AF have streamlined this procedure and greatly increased its utilization. Standardized peri-procedural protocols, represented schematically in Figure 3, improve patient experience, enhance safety, and save on healthcare resources.
Disclosures: The authors have no conflicts of interest to report regarding the content herein. Outside the submitted work, Dr. Gupta has received research funding from Medtronic, Boston Scientific Ltd, and Biosense Webster Ltd, all of whom are manufacturers of products for catheter ablation for AF.
- Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Global rising trends of atrial fibrillation: a major public health concern. Heart. 2018;104:1989-1990.
- Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154-162.
- Bordignon S, Chiara Corti M, Bilato C. Atrial fibrillation associated with heart failure, stroke and mortality. J Atr Fibrillation. 2012;5(1):467.
- Keane D, Ruskin J. Pulmonary vein isolation for atrial fibrillation. Rev Cardiovasc Med. 2002;3(4):167-175.
- Bunch TJ, Cutler MJ. Is pulmonary vein isolation still the cornerstone in atrial fibrillation ablation? J Thorac Dis. 2015;7(2):132-141.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962.
- Bartoletti S, Mann M, Gupta A, et al. Same-day discharge in selected patients undergoing atrial fibrillation ablation. Pacing Clin Electrophysiol. 2019;42(11):1448-1455.
- Celik AI, Kanadasi M, Demir M, et al. Predictors of the paroxysmal atrial fibrillation recurrence following cryoballoon-based pulmonary vein isolation: Assessment of left atrial volume, left atrial volume index, galectin-3 level and neutrophil-to-lymphocyte ratio. Indian Pacing Electrophysiol J. 2019;19(1):9-14.
- Zhuang J, Wang Y, Tang K, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace. 2012;14:638-645.
- Miyazaki S, Kuwahara T, Kobori A, et al. Preprocedural predictors of atrial fibrillation recurrence following pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: long-term follow-up results. J Cardiovasc Electrophysiol. 2011;22:621-625.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-817.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104.
- Kwak J, Pak H, Jang J, et al. Safety and convenience of continuous warfarin strategy during the periprocedural period in patients who underwent catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21(6):620-625.
- Vazquez SR, Johnson SA, Rondina MT. Peri-procedural anticoagulation in patients undergoing ablation for atrial fibrillation. Thromb Res. 2010;126(2):e69-e77.
- De Heide J, Vroegh CJ, Bhagwandien RE, et al. Minimally interrupted novel oral anticoagulant versus uninterrupted vitamin K antagonist during atrial fibrillation ablation. J Interv Card Electrophysiol. 2018;53(3):341-346.
- Martin AC, Godier A, Narayanan K, et al. Management of intraprocedural anticoagulation in patients on non-vitamin K antagonist oral anticoagulants undergoing catheter ablation for atrial fibrillation. Circulation. 2018;138:627-633.
- Chikata A, Kato T, Yaegashi T, et al. General anesthesia improves contact force and reduces gap formation in pulmonary vein isolation: a comparison with conscious sedation. Heart Vessels. 2017;32(8):997-1005.
- Yamaguchi T, Shimakawa Y, Mitsumizo S, et al. Feasibility of total intravenous anesthesia by cardiologists with the support of anesthesiologists during catheter ablation of atrial fibrillation. J Cardiol. 2018;72(1):19-25.
- Martin C, Curtain J, Gajendragadkar P, et al. Improved outcome and cost effectiveness in ablation of persistent atrial fibrillation under general anaesthetic. EP Europace. 2018;20(6):935-942.
- Wasserlauf J, Knight BP, Li Z, et al. Moderate sedation reduces lab time compared to general anesthesia during cryoballoon ablation for AF without compromising safety or long-term efficacy. Pacing Clin Electrophysiol. 2016;39(12):1359-1365.
- Wynn GJ, Haq I, Hung J, et al. Improving safety in catheter ablation for atrial fibrillation: a prospective study of the use of ultrasound to guide vascular access. J Cardiovasc Electrophysiol. 2014;25:680-685.
- Haegeli LM, Duru F, Lockwood EE, et al. Feasibility and safety of outpatient radiofrequency catheter ablation procedures for atrial fibrillation. Postgrad Med J. 2010;86:395-398.
- Opel A, Mansell J, Butler A, et al. Comparison of a high throughput day case atrial fibrillation ablation service in a local hospital with standard regional tertiary cardiac centre care. Europace. 2018;21:440-444.
- Kuck KH, Fürnkranz A, Chun KR, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37(38):2858-2865.
- Brito V G, N V, L T, et al. Second generation cryoballoon vs. radiofrequency ablation in paroxysmal atrial fibrillation: outcomes beyond one-year follow-up. J Atr Fibrillation. 2019;11(6):2147.
- Cardoso R, Mendirichaga R, Fernandes G, et al. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2016;27(10):1151-1159.
- Kautzner J, Neuzil P, Lambert H, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17(8):1229-1235.
- Das M, Loveday J, Wynn G, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. EP Europace. 2017;19(5):775-783.
- Wakamatsu Y, Nagashima K, Watanabe I, et al. The modified ablation index: a novel determinant of acute pulmonary vein reconnections after pulmonary vein isolation. J Interv Card Electrophysiol. 2019;55(3):277-285.
- Hussein A, Das M, Riva S, et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients. Circ Arrhythm Electrophysiol. 2018;11(9):e006576.
- Lee SR, Choi EK, Lee EJ, Choe WS, Cha MJ, Oh S. Efficacy of the optimal ablation index-targeted strategy for pulmonary vein isolation in patients with atrial fibrillation: the OPTIMUM study results. J Interv Card Electrophysiol. 2019;55(2):171-181.
- Taghji P, El Haddad M, Phlips T, et al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol. 2018;4(1):99-108.
- Reichlin T, Knecht S, Lane C, et al. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm. 2014;11(2):194-201.
- Winkle RA, Moskovitz R, Hardwin Mead R, et al. Atrial fibrillation ablation using very short duration 50 W ablations and contact force sensing catheters. J Interv Card Electrophysiol. 2018;52(1):1-8.
- Chen S, Schmidt B, Bordignon S, et al. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J Cardiovasc Electrophysiol. 2019 Oct 6.
- Hussein A, Das M, Chaturvedi V, et al. Prospective use of ablation index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28(9):1037-1047.
- Aryana A, Kenigsberg DN, Kowalski M, et al; Cryo-DOSING Investigators. Verification of a novel atrial fibrillation cryoablation dosing algorithm guided by time-to-pulmonary vein isolation: results from the Cryo-DOSING study (Cryoballoon-ablation DOSING Based on the Assessment of time-to-effect and pulmonary vein isolation guidance). Heart Rhythm. 2017;14(9):1319-1325.
- Han HC, Ha FJ, Sanders P, et al. Atrioesophageal fistula: clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol. 2017;10(11).
- Knopp H, Halm U, Lamberts R, et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm. 2014;11(4):574-578.
- Halm U, Gaspar T, Zachäus M, et al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2010;105(3):551-556.