ADVERTISEMENT
Starting a His Bundle Pacing Program: Our Experience at Rush University Medical Center
Introduction
With emerging data on its benefits over conventional RV pacing, permanent His bundle pacing (HBP) has gained significant traction over the past few years.1-3 There are also emerging data on the benefits of HBP for cardiac resynchronization therapy.3-7
Approximately three years ago, we started an HBP program at Rush University Medical Center (Rush), an academic medical center with over 650 beds. Since then, we have performed over 270 HBP implants, with a greater than 95% success rate. In this article, we discuss key considerations to starting a successful HBP program, with a focus on HBP device follow-up.
Education: Providers, Peers, and Patients
Continuing education and effective communication are crucial in building a successful program. This includes continued education for physicians, electrophysiology (EP) lab staff, device clinic staff, and industry. At Rush, the nurses who support cases in the EP lab also perform follow-up device checks in device clinic. This unique model provides our nurses with the opportunity to learn during the implant as well as follow these patients in the device clinic.
As physicians, we must educate referring providers regarding the benefits and challenges associated with this technique. Educating radiology colleagues reading the post-procedure chest x-ray (CXR) is key. Experience has shown that mentioning “HBP lead” on the CXR orders helps our radiology colleagues to identify these leads. Patient education is also critical, particularly if a patient visits a facility where the healthcare team is not familiar with HBP. For example, one of our patients was treated at an outside hospital and advised that she would need a lead revision. Fortunately, because she had been educated on HBP, the patient was able to act as her own advocate. The patient provided the care team with our contact information at Rush, which spared the patient from receiving an unnecessary lead revision.
HBP Terminology
Over the past three years, the terminology and programming for HBP have continued to evolve. To ensure effective communication, it is important that healthcare providers speak the same language. With a lead implanted at the His bundle (HB) location, the paced morphology could be nonselective capture (NS-HBP), which represents capture of the HB and the surrounding ventricular myocardium, and/or selective capture (S-HBP), which represents capture of the HB alone (Figure 1).8 NS-HBP is identified by presence of a pseudo delta wave, or “slur”, leading into a narrow QRS complex. S-HBP is identified by an isoelectric, or “flat straight line”, preceding the QRS complex. Figure 1 demonstrates the transition from NS-HBP to S-HBP on a 12-lead rhythm strip. The type of HB capture is determined by multiple factors, including the patient’s anatomy and the level of conduction disease. These changes in morphology should be assessed at implant and during follow-up using 12-lead rhythm strips while decrementing the pacing output. Educating the nursing staff on these concepts and reviewing 12-lead rhythm strips with the staff is important to ensure consistency in reporting.
Implantation and Follow-Up
Patience and persistence are key to improving experience and success rates with HBP. When implementing an HBP program, we recommend starting with straightforward cases, such as patients with normal heart size with sinus node dysfunction or AV nodal block,8 before advancing to more complicated cases of advanced AV block, such as infra-nodal disease or bundle branch block (BBB) patterns. For the implanting physician, it is also important to know the tools that are currently available. To enhance the learning experience and accelerate program implementation, invite EP lab support staff to participate in your cases.
During follow-up, careful evaluation of the following parameters is also important:
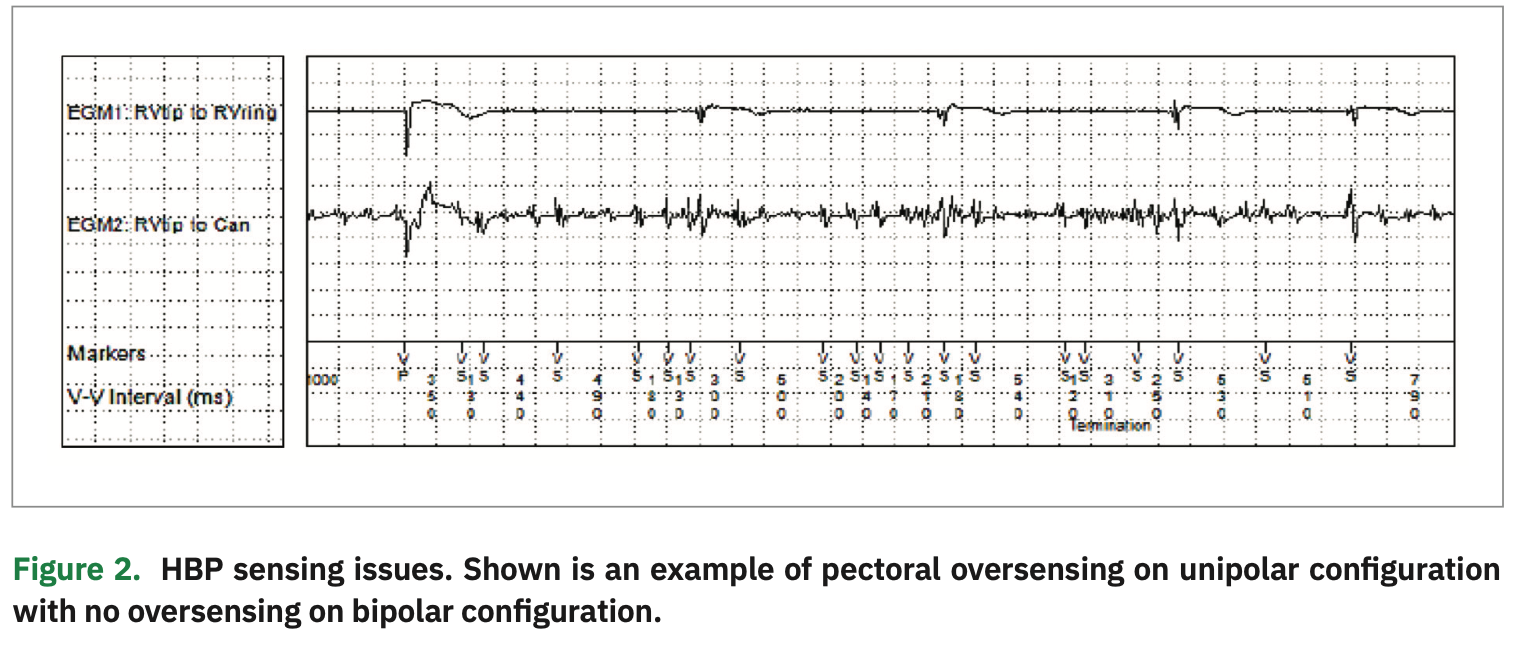
Sensing: Sensing is best assessed at time of implant prior to permanently fixing the lead. An acceptable R-wave amplitude for a lead in the HB location is >1 mV, unless the patient is pacing dependent. Here, understanding the sensitivity limitations of a pulse generator is important. For example, the sensitivity on the Adapta device (Medtronic) can be programmed as low as 1 mV, whereas the Advisa and Azure (Medtronic) allow programable sensitivity as low as 0.45 mV. The R-wave sensing is usually higher in the unipolar configuration. However, we caution against programming unipolar sensing, especially in patients who are dependent due to the risk of myopotentials and oversensing, which can lead to possible inhibition of pacing. As shown in Figure 2, the unipolar sensing electrogram (EGM) reveals noise due to pectoral myopotential oversensing, while the bipolar configuration does not exhibit oversensing. It is also critical to pay attention to atrial signal amplitude on the His channel at implant, which is more common in the bipolar configuration. Frequently, staff can program around this issue by adjusting the sensitivity of the HB lead, making it less sensitive.
Threshold testing: When checking HBP thresholds, we prefer that testing be done in VVI mode to avoid pseudofusion that can occur with inappropriately programmed AV delays. Threshold testing should always start at 5V@1ms while decrementing outputs and monitoring for changes in paced morphology.
Access to a 12-lead ECG and running rhythm strips during threshold testing is critical and can be challenging for new HBP programs. Start by obtaining a presenting rhythm strip. As long as the patient is not dependent, obtain an underlying rhythm strip to assess the patient’s native QRS, to assess if the underlying QRS is narrow or if there is an underlying BBB. Threshold checks should be performed in both bipolar and unipolar configurations. During the threshold test, run the 12-lead rhythm strip for the entire duration to assess for changes in morphology and note the output at which these changes occur.
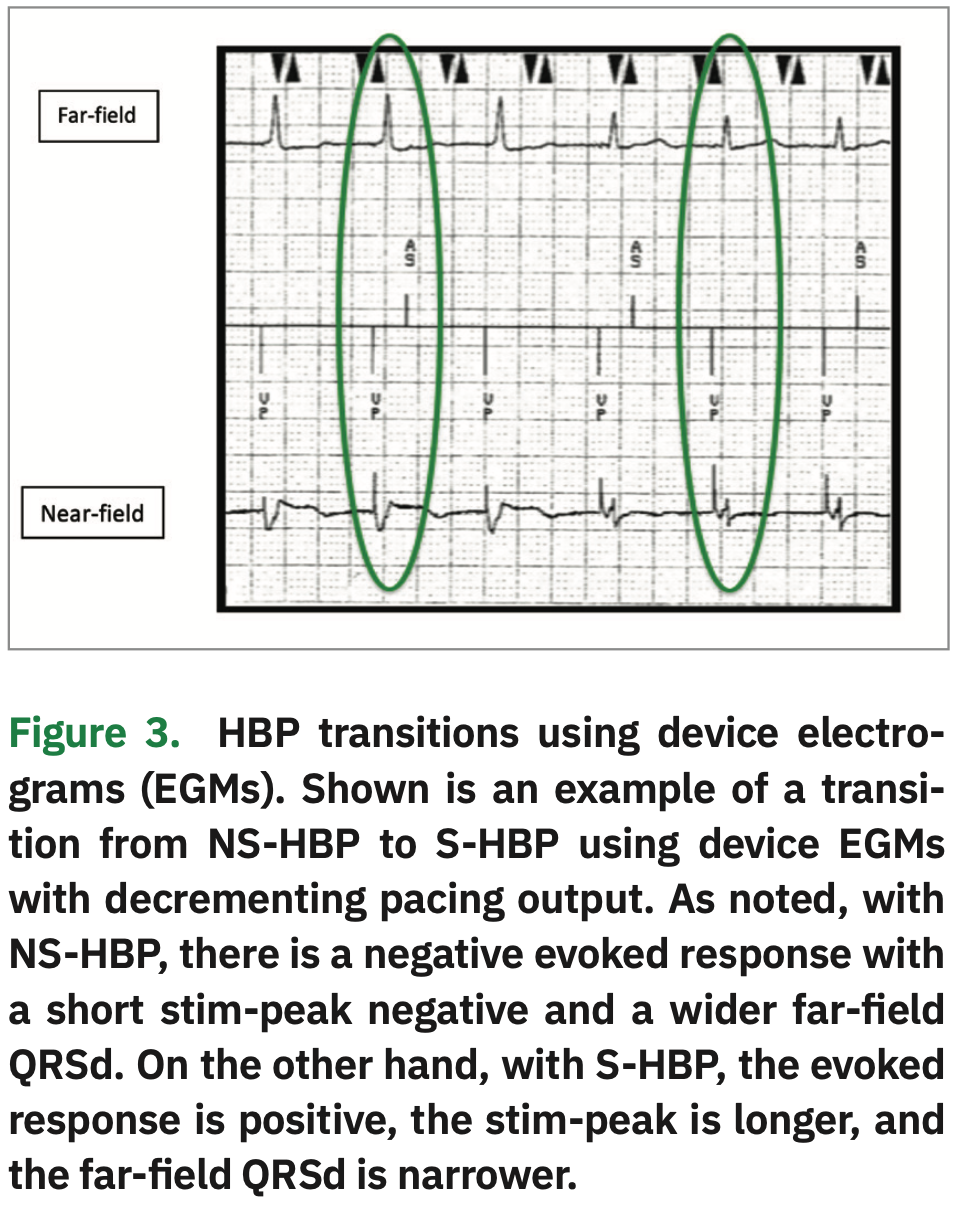
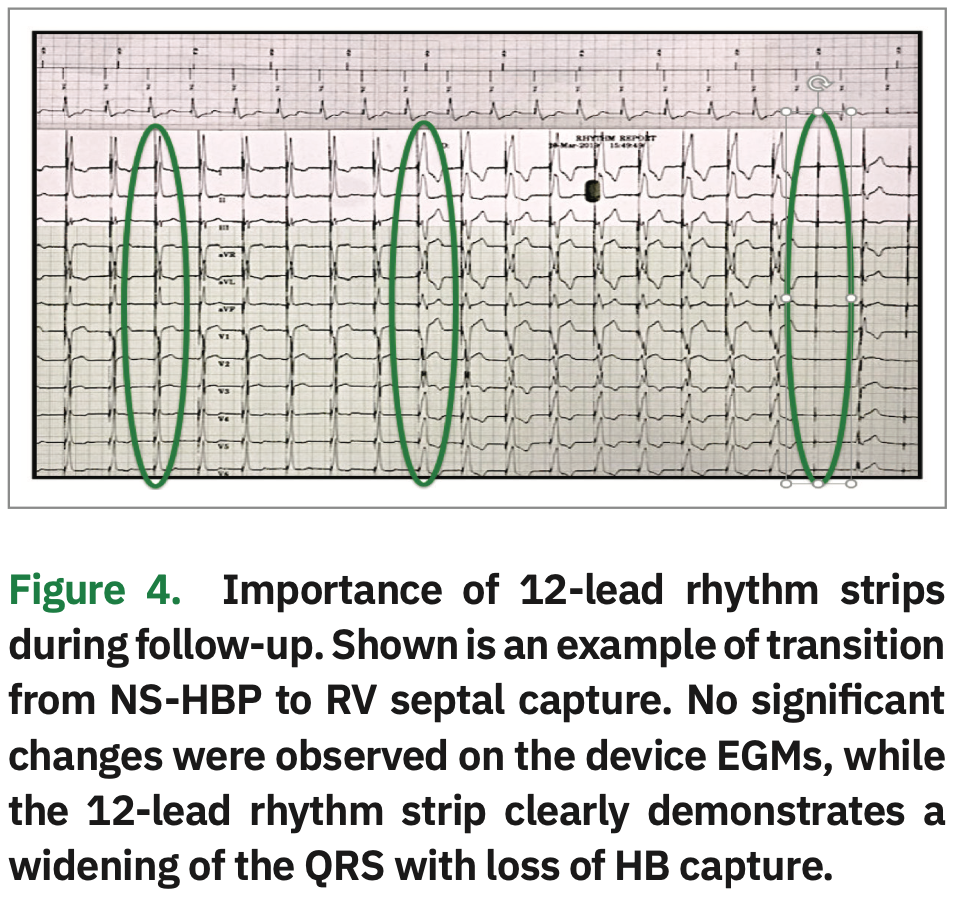
Device EGMs can also be used to assess morphology transitions along with 12-lead rhythm strips.9 When looking at the near-field EGM, an initial negative deflection is indicative of NS-HBP, and an initial positive deflection is indicative of S-HBP. “Time to peak” (ie, time from the pacing spike to the onset of the QRS complex) is shorter for NS-HBP, because the lead captures both RV septal and His tissue. Time to peak is longer for S-HBP because of the time it takes for the impulse to travel down the His-Purkinje axis to the ventricle. When using the far-field EGMs, the QRS is often wider in NS-HBP and narrower with S-HBP (Figure 3). However, staff must not rely solely on device EGMs for transitions. In Figure 4, there is no change in morphology shown on the device EGMs. However, on the 12-lead rhythm strip, a change in morphology from NS-HBP to RV septal capture is evident. We recommend that a 12-lead rhythm strip be done at implant, post-op day #1, four weeks post implant, three months post implant, and as needed.
Programming for HBP: At Rush, we have established the following standard protocol for programming HBP devices. During the first three months post implantation, the programmed output is set at 5V@1ms regardless of the threshold. At the three-month check, the threshold can be checked at 0.4 ms pulse width provided that: (1) the HB threshold fluctuations have remained <1V; (2) the patient is not dependent; and (3) the His capture threshold or BBB recruitment threshold is less than 1V@1ms. If the threshold is less than 1.5V@0.4ms, the pulse width is decreased to 0.4 ms to promote battery longevity. We generally program the His lead output 1V above HB capture threshold if the patient has a narrow QRS, or 1V above BBB correction threshold if the patient has a BBB at baseline. The absolute minimum output should be 2V@0.4ms. Capture management for the His lead is programmed to monitor only or off due to potential inability of the device to appropriately detect or differentiate an evoked response during HB capture.
If a patient is implanted for sinus node dysfunction, the device is programmed in a DDDR mode with prolonged AV delays. If a patient is implanted for AV block, the device is programmed in a DDD mode with short AV delays. The AV delays are shortened to accommodate for HV interval and may vary patient to patient based on measurements taken at implant.
Challenges with follow-up: HBP device checks can be time consuming. Allowing for a longer clinic appointment slot may be necessary, especially when the team members checking the devices are still familiarizing themselves with HBP. Running the 12-lead rhythm strip can add a significant amount of time to the device check. In addition, if the device clinic is large and very busy, it may be difficult to find an open appointment slot four weeks post implant. Advance planning for these operational changes will help to ensure a successful HBP program. When checking HBP, there are challenging concepts that need to be understood. Programming and troubleshooting can be overwhelming at first. Therefore, to assist device clinic nurses when checking HBP devices, we have also developed a HBP tip sheet that focuses on programming and troubleshooting.
Acknowledgements: We thank the Rush University Medical Center EP team, and in particular Dr. Richard Trohman (Director of Electrophysiology Division), for their team effort and dedication to making the Rush HBP program a success.
Disclosures: Ms. Hanifin reports honoraria from Medtronic. Dr. Sharma reports honoraria from Medtronic; he is also a consultant for Medtronic, Abbott, Boston Scientific, and BIOTRONIK.
- Sharma PS, Dandamudi G, Naperkowski A, et al. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;12(2):305-312.
- Abdelrahman M, Subzposh FA, Beer D, et al. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71:2319-2330.
- Sharma PS, Vijayaraman P, Ellenbogen KA. Permanent His bundle pacing: shaping the future of physiological ventricular pacing. Nat Rev Cardiol. 2019 Jun 27. [Epub ahead of print]
- Sharma PS, Dandamudi G, Herweg B, et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15(3):413-420.
- Sharma PS, Naperkowski A, Bauch TD, et al. Permanent His bundle pacing for cardiac resynchronization therapy in patients with heart failure and right bundle branch block. Circ Arrhythm Electrophysiol. 2018;11(9):e006613.
- Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, et al. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: a crossover design comparison. Heart Rhythm. 2015;12(7):1548-1557.
- Upadhyay GA, Vijayaraman P, Nayak HM, et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol. 2019;74(1):157-159.
- Vijayaraman P, Dandamudi G, Zanon F, et al. Permanent His bundle pacing: recommendations from a multicenter His bundle pacing collaborative working group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018;15(3):460-468.
- Saini A, Serafini NJ, Campbell S, et al. Novel method for assessment of His bundle pacing morphology using near field and far field device electrograms. Circ Arrhythm Electrophysiol. 2019;12(2):e006878.