Intraoperative Epicardial Voltage Mapping to Guide Hybrid Convergent Ablation of Persistent Atrial Fibrillation
Introduction
Atrial fibrillation (AF) ablation has evolved over the past 30 years into an effective treatment option for patients with AF.1 Catheter and surgical approaches have similar success rates, but differ in their technical advantages and disadvantages.1 The convergent procedure is composed of two steps: surgical epicardial radiofrequency (RF) ablation of the posterior left atrial (LA) wall, followed by percutaneous endocardial RF catheter ablation to isolate the pulmonary veins. This strategy has similar success rates to endocardial ablation alone, and also may minimize the risk of ablating the endocardial LA posterior wall near the esophagus.2-4
Epicardial ablation is performed through a subxiphoid incision, with a videoscope and ablation tool inserted through a large-bore trocar into the pericardial space. This allows partial visualization of the inferior posterior wall.3 This step is considered a “blind” ablation, because the surgeon cannot visualize the entire posterior wall or evaluate the completeness of the ablation with electroanatomical mapping. Confirmation of voltage or demonstration of entrance or exit block is the standard of care, and is performed after percutaneous endocardial ablation by electrophysiologists.
In this article, we report a case of convergent surgical ablation during which we performed epicardial electroanatomical mapping before and after ablation to guide the surgeon, and to demonstrate the effectiveness of the procedure with minimal additional procedure risk.
Case Description
The patient is a 52-year-old male with persistent atrial fibrillation refractory to multiple antiarrhythmic drugs. He remained symptomatic with rapid ventricular rates, and elected to undergo hybrid convergent ablation.
At the start of the procedure, an incision was made below the xiphoid process, and the convergent trocar was advanced into the pericardial space. A videoscope was used to identify the pulmonary veins and coronary sinus, determining the lateral and inferior borders of the ablation. An Advisor HD Grid Mapping Catheter, Sensor Enabled (Abbott) was inserted through the trocar into the pericardial space, and was used to determine LA posterior wall geometry and tissue bipolar voltage. Findings were recorded and displayed on the EnSite Precision Cardiac Mapping System (Abbott).
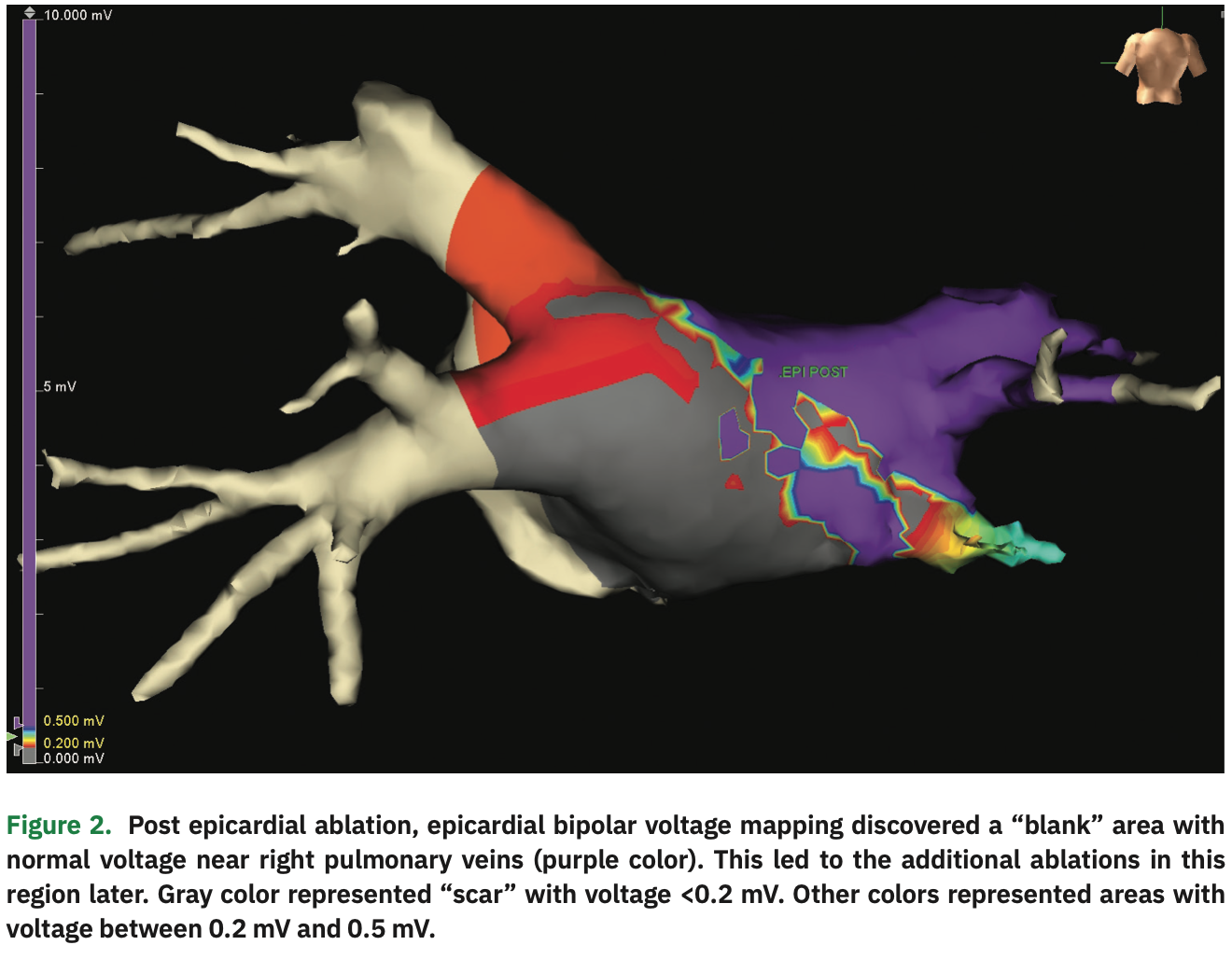
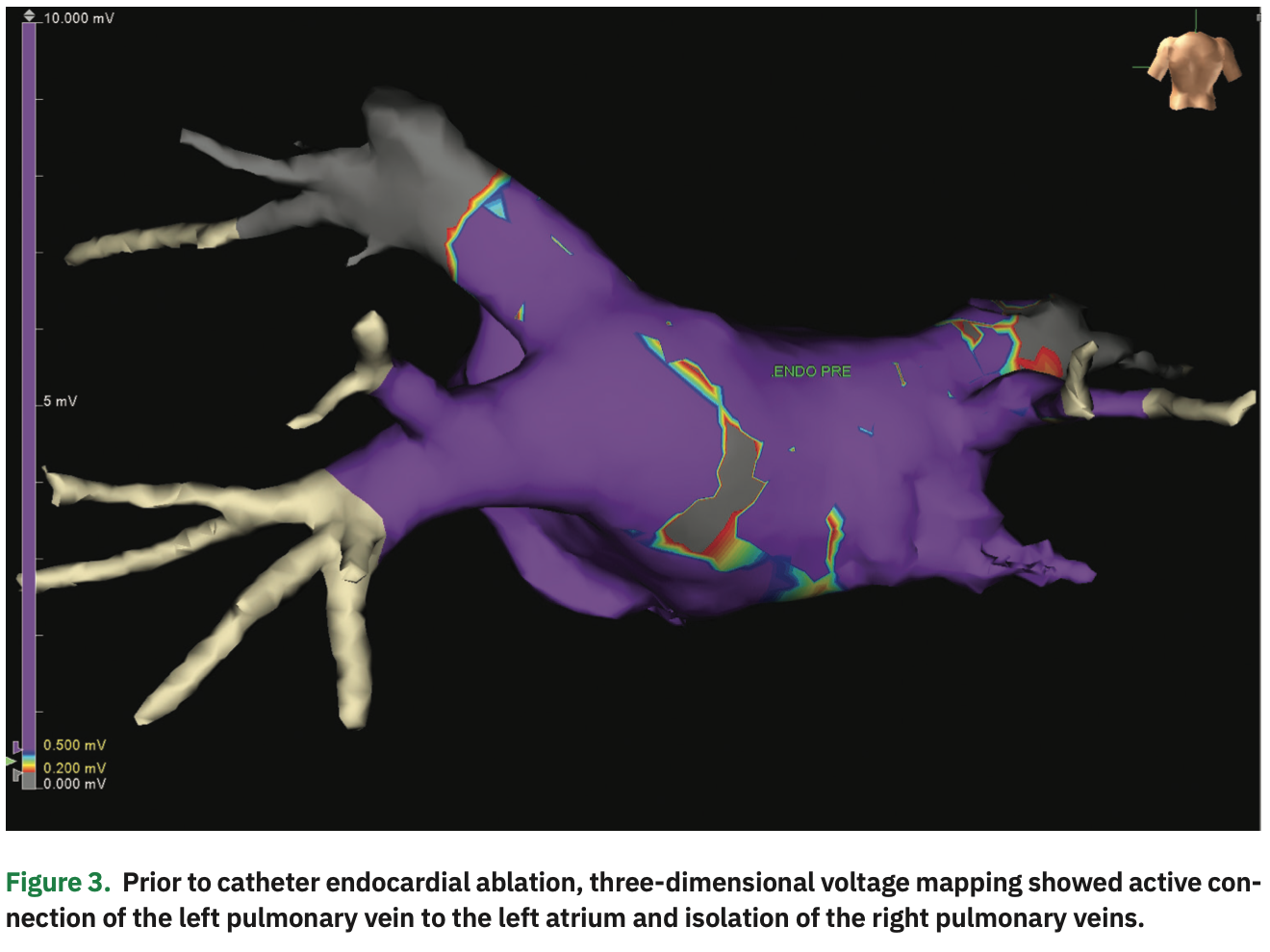
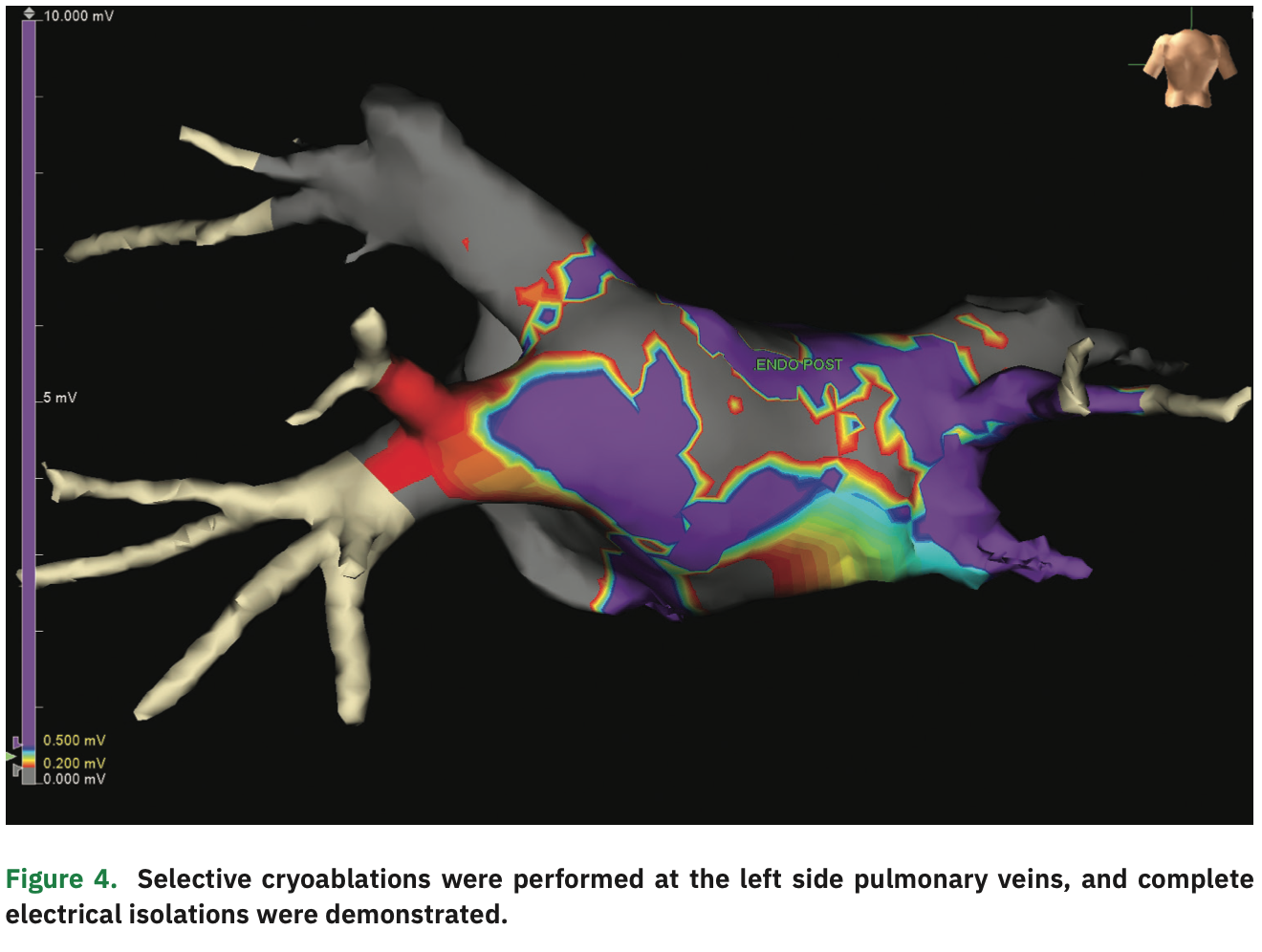
The initial bipolar voltage amplitude was >0.5 mV throughout the posterior wall, in the normal range (Figure 1). An EPi-Sense unipolar RF catheter (Atri-Cure, Inc.) was then inserted into the pericardial space, and 28 separate ablations were performed on the surface of LA posterior wall. Post ablation, the Advisor HD Grid was re-inserted to generate a second voltage map. “Scar” (voltage <0.2 mV) was demonstrated in most of the posterior wall, but there was still an area with normal voltage signals (>0.5 mV) near the right pulmonary veins (Figure 2). Under the guidance of the voltage map, additional epicardial ablations were performed in the normal voltage area until low voltage was observed throughout the posterior wall. After the conclusion of epicardial ablation, percutaneous endocardial AF ablation was performed with the Arctic Front cryoballoon system (Medtronic) in a standard fashion. Pre-ablation endocardial electroanatomical mapping revealed electrically active left pulmonary veins and electrical isolation of the right pulmonary veins (Figure 3). Percutaneous cryoballoon ablation was then performed at the left pulmonary vein antra, with an endpoint of electrical isolation (Figure 4). The patient has been free of atrial fibrillation and off antiarrhythmic drugs since the procedure.
Discussion
Catheter ablation of persistent AF is more challenging compared to paroxysmal AF, frequently requiring substrate modification in addition to pulmonary vein antral isolation.5 Isolation of the LA posterior wall remains difficult due to the need for long linear lesions or dense ablation between the PV antra in close proximity of the esophagus. Esophageal temperature monitoring during ablation may also help avoid dangerous esophageal temperature rises and prevent ulcers or fistula formation, but with increased chance of insufficient ablation near the esophagus.6
During epicardial ablation, surgeons experience limited visualization through the videoscope and may fail to reach the entire posterior wall near the pericardial reflections.1 In addition, high-density mapping of the epicardial side of the left atrium pre- and post-ablation is often not performed.6 An intraoperative epicardial electroanatomical map can provide the surgeon with instantaneous feedback to improve ablation effectiveness and reduce epicardial “blind spots.” During our case, an incompletely treated area with normal voltage was discovered next to the right pulmonary veins by post-ablation epicardial mapping, which led the surgeon to perform additional ablations in that area. This highlights the advantages of intraoperative epicardial electroanatomical mapping during surgical ablation, with minimal additional procedure risks.
Conclusion
An epicardial electroanatomical voltage map can improve ablation effectiveness and completeness during the convergent procedure. We believe this interdisciplinary, collaborative team approach may result in a higher success rate for AF patients; however, randomized controlled trials are needed to demonstrate the true efficacy of epicardial mapping during the convergent procedure.
Disclosures: The authors have no conflicts of interest to report regarding the content herein.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
- Kiser AC, Landers M, Horton R, et al. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum. 2010;13(5):E317-E321.
- Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60(1):54-61.
- van der Heijden CAJ, Vroomen M, Luermans JG, et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2019;56(3):433-443.
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812-1822.
- Kumar P, Bamimore AM, Schwartz JD, et al. Challenges and outcomes of posterior wall isolation for ablation of atrial fibrillation. J Am Heart Assoc. 2016 Sep 23;5(9).