Financial Disincentives for Physicians to Implant the Subcutaneous Defibrillator
It is surprising how many young patients who have an indication for an implantable cardiac defibrillator (ICD) still receive a transvenous defibrillator when the long-term risks of transvenous leads in young patients are well known, and when a totally subcutaneous implantable defibrillator (S-ICD) has been a commercially available option for these patients for several years.1-2 There were initial concerns that the S-ICD might not be as effective as the transvenous ICD. Barriers to more widespread adoption have included the larger generator size, placement in a nontraditional location, continued perception of a high rate of inappropriate shocks, the inability to deliver antitachycardia pacing, and the requirement for at least 2 incisions, tunneling of the lead, and defibrillation testing often translating to the need for deeper sedation or general anesthesia. In addition, despite several years since FDA approval, some insurance companies continue to deny preauthorization for the S-ICD.3 However, there have now been several published studies demonstrating the safety and efficacy of the S-ICD. A recent randomized clinical trial, the PRAETORIAN trial,4 showed in 849 patients that the S-ICD was at least comparable to a transvenous system with a 79% reduction in lead-related complications, and comparable mortality after 4 years. The recently published UNTOUCHED trial5 showed a 68% reduction in inappropriate shocks with contemporary S-ICD software and programming. It is hopeful that this new information might lead to wider adoption.
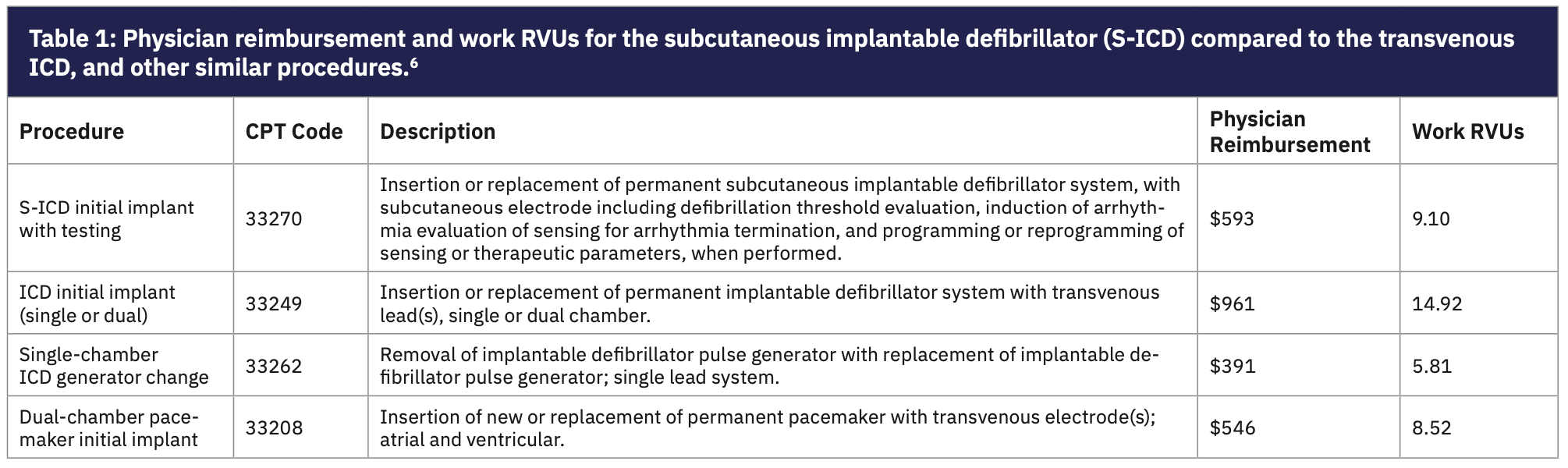
Unfortunately, there remains a critical financial roadblock to more appropriate utilization of the S-ICD. That roadblock is insufficient physician reimbursement for the procedure. The Centers for Medicare & Medicaid Services (CMS) physician reimbursement for implantation of a S-ICD transvenous defibrillator implant is $593 with a work RVU value of 9.10 (Table 1).6 This is considerably lower than the reimbursement for implantation of a transvenous defibrillator at $961 and 14.92 RVUs. This difference is in spite of bundling of payments that include defibrillation testing with the S-ICD. Reimbursement for the S-ICD is closer to that of a single-chamber ICD generator change at $391, and is comparable to reimbursement for implantation of a standard pacemaker.
Physician payment by CMS MD signed RVUs are based on a complex reimbursement assignment. The determinations consider the amount of time involved to prepare for and perform the procedure, but also involves difficulty and risk. It is hard to make a case that implantation of an S-ICD with induction of ventricular fibrillation and device testing should be associated with a reimbursement to the physician of only $200 more than reimbursement for a simple defibrillator generator change.
There are several reasons why the totally subcutaneous defibrillator accounts for a small proportion of single-chamber ICD implants. Recent publication of the PRAETORIAN and UNTOUCHED trials should provide a strong case for more appropriate use. An argument could be made, however, that appropriate candidates may still not receive the device because physician reimbursement for implantation of the S-ICD is unjustifiably low. This financial disincentive should be reevaluated.
Stay safe,
Bradley P. Knight, MD, FACC, FHRS
@DrBradleyKnight
Editor-in-Chief, EP Lab Digest
Disclosures: Dr. Knight reports that he is a consultant, speaker, investigator, and offers fellowship support for Abbott, Baylis Medical, Biosense Webster, Inc., BIOTRONIK, Boston Scientific, Medtronic, and SentreHEART. He has received compensation for serving as a consultant to CVRx, Inc.
- Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36-44.
- Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605-1615.
- Knight, BP. Delay in insurance preauthorization for a defibrillator nearly costs patient with hypertrophic cardiomyopathy her life. EP Lab Digest. 2019;19(12):4.
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383:526-536.
- Gold MR, Lambiase PD, El-Chami MF, et al; UNTOUCHED Investigators. Primary results from the Understanding Outcomes with the S-ICD in Primary Prevention Patients with Low Ejection Fraction (UNTOUCHED) Trial. Circulation. 2020 Oct 19. doi: 10.1161/CIRCULATIONAHA.120.048728. Epub ahead of print.
- Fall 2020 Procedural Payment Guide. Boston Scientific. Available at https://bit.ly/36vrru1. Accessed November 11, 2020.