Expanding the Pediatric Cardiac Monitoring Tool Box: Experience With Novel Ambulatory Cardiac Rhythm Monitoring Technologies
Introduction
Cardiac arrhythmia is a common cause of morbidity in children, especially children born with congenital heart disease. Therefore, early and accurate detection may prevent life-threatening consequences. Traditionally, Holter monitors have been used as the go-to tool for ambulatory electrocardiogram (ECG) monitoring to evaluate cardiac arrhythmias. However, their bulky design leads to low user satisfaction and compliance in children.1 Recent innovations in ambulatory ECG monitoring technology have significantly improved both patient comfort and diagnostic yield. For example, wearable cardiac monitoring devices have allowed the combination of two essential characteristics of an effective ambulatory monitoring system: long-term continuous heart rate and rhythm monitoring, and on-demand ECG tracing capture.
These new technologies and devices have expanded the physician’s monitoring tool box, allowing them to choose the most appropriate tool depending on each patient’s diagnosis. Reliance on telemedicine and ambulatory monitoring has become even more apparent to ensure patient safety during the current COVID-19 pandemic. Here, we detail our experience with some of these innovative technologies.
Smartphone-Enabled ECG Devices
Technology of devices such as the KardiaMobile (AliveCor, Inc.) can generate diagnostic single-lead real-time ECG tracings recorded on a smartphone and transmitted wirelessly to the cloud. Studies in children have found that the device can generate a high percentage of diagnostic ECG tracings (96%) and had better diagnostic yield than conventional patient-activated event monitors (25% vs 6%).2,3 Also notable is that user satisfaction, both from parents and children, is positive regarding recording, transmission, and comfort while using the device.2 More recently, the addition of another electrode to the device to allow it to record a 6-lead ECG tracing further expands the device’s capability, but its usability in children remains to be seen. Possible limitations of these smartphone-based devices include the potential for increased motion artifact when used in young children. Moreover, the need to have the sensor connected to the app may increase the lag time to capture the rhythm and diagnosis. It would be interesting to compare the diagnostic capabilities and user satisfaction between the app-based devices to the standalone devices, in which the sensor, screen, and recording software are all incorporated in one device.
Single-Lead ECG Patches
Single-lead ECG patches such as the Zio XT (iRhythm Technologies) have been used for ambulatory ECG monitoring, maintaining signal quality for up to 14 days. Although existing in only one size, the devices are small and unobtrusive enough to be used in infants as young as 3 weeks old.4 In our anecdotal experience, we have been able to use them in small infants and even children with a chest deformity. When compared directly to Holter monitoring in children, the Zio has been found to perform as well as the Holter in terms of diagnostic yield.4,5 Seventy-five percent of children, young adults, and parents preferred the Zio over the Holter, since the Zio had no wires and allowed them to shower.4 Also notable is that in one study, the majority of the arrhythmias diagnosed in the Zio group occurred after 24 hours.5 These arrhythmias would have been missed with the conventional Holter.
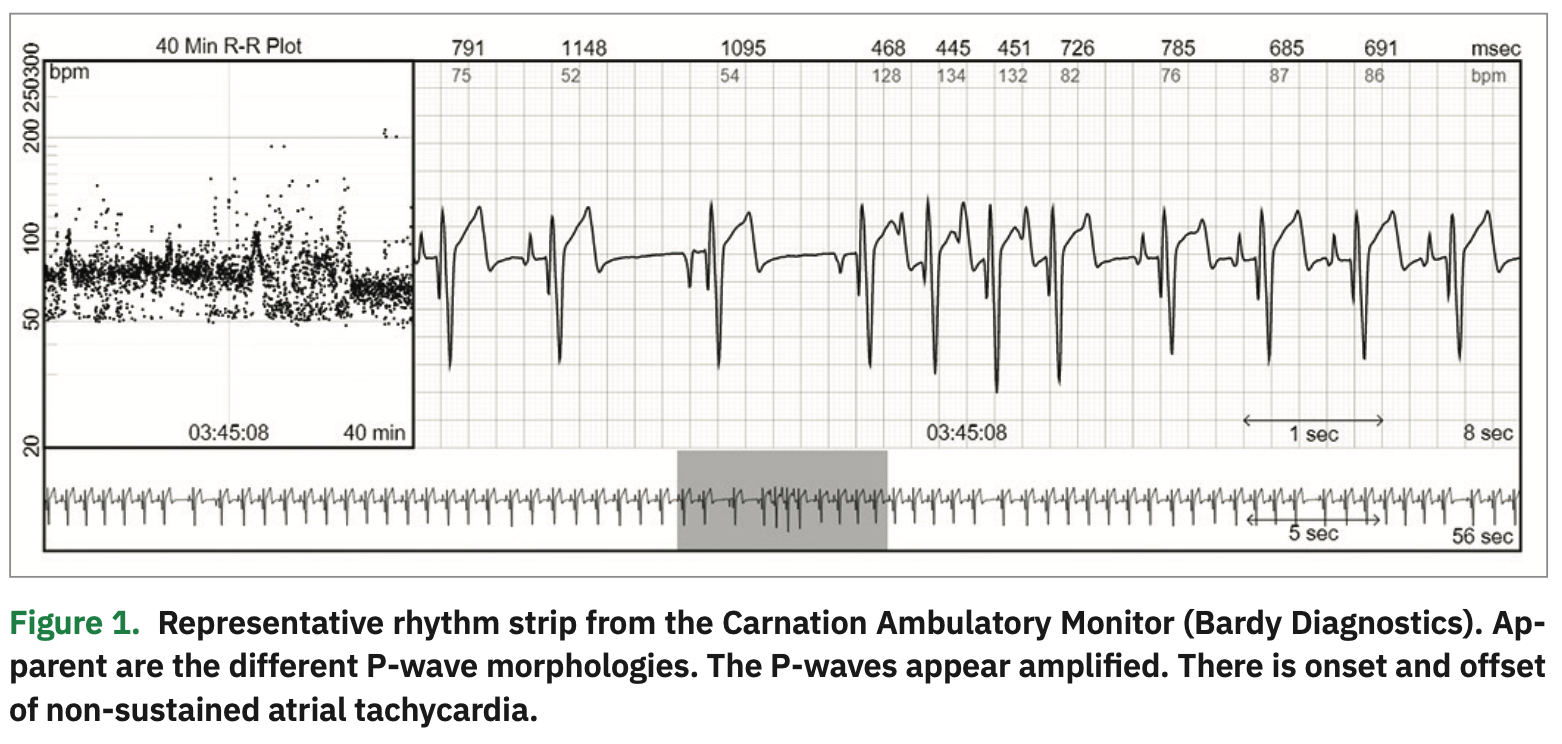
A recent improvement in the single-lead ECG patch technology is the P-wave centric monitor. One example is the Carnation Ambulatory Monitor (CAM) Patch (Bardy Diagnostics), which uses a novel circuit design and advanced compression algorithms to process the ECG signal, producing a high-clarity ECG signal that is P-wave centric.6 The device comprises wireless electrodes worn in-line with the sternum similar to the ES lead of the EASI system.7 In our experience, the slender, lightweight design of the device increased patient comfort when worn and is often unnoticeable underneath a shirt. The over-the-sternum placement is especially accommodating of the female chest anatomy. P-waves were often amplified, making them more clearly visible without incurring excessive noise. The clarity of the atrial activity as recorded by the CAM device has helped us in the diagnosis and management of both atrial and ventricular arrhythmias in pediatric patients. Figure 1 presents an example of a rhythm strip showing amplified varied P-wave morphologies as well as onset and offset of non-sustained atrial tachycardia. Head-to-head comparison between the CAM and Holter monitors has not yet been performed.
Another advantage of the single-lead ECG monitors is the ability for patients to initiate monitoring from the comfort of their home. Many monitoring companies offer a mail-to-patient option, liberating the patient from needing to have an in-person clinic visit. On the other hand, the turnaround time for analysis can be delayed since the devices need to be returned to the manufacturer or third party for processing. Engaging in provider self-scanning of data using web-based ECG analysis modules provided by the monitor companies could help make the process more efficient and allow immediate access to the important full-disclosure raw data. From a user experience standpoint, certain patches require the skin to be abraded prior to application of the patch, which may cause discomfort to the young patients. Finally, the diagnostic algorithms and detection zones of these devices may have to be adjusted for children where high-rate SVT is a frequent diagnosis.
Mobile Cardiac Telemetry Patches
A monitoring technology with a high diagnostic yield is mobile cardiac outpatient telemetry (MCOT) such as the MCOT Patch developed by BioTelemetry.8 The single-lead patch device can continuously record and transmit data leading to real-time diagnosis. Continuous and real-time diagnosis is useful in symptomatic or high-risk patients. For example, we recently prescribed MCOT to monitor an infant with a cardiac tumor that was causing ventricular arrhythmias. The MCOT system allowed for near real-time monitoring of the arrhythmia burden and cardiac rhythm during initiation of beta-blockade therapy and titration from home. The system allowed for a limit on the frequency of in-person visits and may have decreased the risk of infection of COVID-19. MCOT can also be used in an inpatient setting, helping to free up telemetry beds. Recently, MCOT has been used to monitor QT prolongation in COVID-19 patients treated with hydroxychloroquine and/or azithromycin on a non-telemetry floor.9 Moreover, the MCOT system allows near real-time notification of any arrhythmias to the electrophysiologists, leading to prompt assessment and management of the patients.9 MCOT has not been approved to measure and monitor QTc during atrial fibrillation/flutter, or when the QRS is greater than 160 ms, which could limit its application in patients with congenital heart disease. Finally, addition of features such as the ability to view the rhythm live on the smartphone for the user and web-based reporting platform for the physician may enhance user satisfaction and engagement while removing geographical barriers to the use of this technology.
On-Demand 12-Lead ECG
While patch ECG technologies have become more advanced, 12-lead ECG remains the gold standard for non-invasive cardiac electrophysiologic diagnosis. Improvements have steadily been made to the form factor (smaller and lighter) and to the electrodes. QT ECG (QT Medical), a true 12-lead ECG device, is one of the first FDA-approved, medical-grade, portable, and wireless 12-lead ECG devices that also allows for general consumer use, as the device can be mailed to the consumer.10 The system is comprised of an ECG recorder, the PCA 500, and pre-shaped electrodes of various sizes. Specifically, there are 3 pediatric sizes and 3 adult sizes. The manufacturer recommends using the pediatric Size 1 for children <1 year old, pediatric Size 2 for children between 1 and 4 years old, and pediatric Size 3 for children between 5 and 10 years old. Size S for adults could be used for children aged 11 years and older. The PCA 500 records the ECG at a sampling rate of 1000 Hz, making it suitable for use in both adults and children. The pre-shaped and pre-positioned electrodes allow for easy, accurate, and consistent electrode placement, even by the lay person.11 The system is complemented by cloud-based ECG storage and interpretation application, making it an ideal tool for use in telemedicine. Currently, we are employing this technology on our pediatric patients enrolled in investigational drug studies who cannot come on campus. The home use of QT ECG to obtain ECGs has successfully avoided disruptions in care and breach of study protocol. On one such patient, the ECG showed new onset of T wave abnormalities, and the patient was referred for further evaluation (Figure 2). In our experience, it took the family/patient less than 3 minutes to obtain an ECG and have it available for review. The versatility of this device could be deployed in other diagnostic areas such as health screenings in schools, athletic events, outreach clinics, or during exercise stress testing.
While the sampling rate of 1000 Hz and 150 Hz bandwidth is deemed adequate for use in children, previous studies have advocated for at least a sampling rate of 1200 Hz with a 250 Hz bandwidth to be used to acquire and process ECG signals in children.12,13 Moreover, the use of pre-shaped electrodes precludes their use in situations where the heart may be displaced in the thoracic cavity such as dextrocardia, or when there is unusual chest anatomy, especially in children with congenital heart disease. Finally, while the device currently has several quality checkpoints (missing lead, low voltages) to ensure good ECG quality, it does not yet have the automatic excessive noise and artifact detection algorithm that may be important when acquiring an ECG in uncooperative infants and young children.
Discussion
These described digital technologies have quickly reshaped how we monitor cardiac rhythm. Yet, the future of cardiac rhythm monitoring technology will undoubtedly feature smaller and less intrusive sensors and devices (textile sensors, biological sensors) with longer battery life that continuously collect data. We will have to pursue the development of artificial intelligence (AI) to automatically and accurately process this increasingly vast amount of data, freeing up physicians to spend more time treating and counseling their patients. Currently, machine learning and deep learning-based algorithms can read ECGs as well as a seasoned human reader.14 Additionally, they can also predict events based on patterns not readily seen by the naked human eye.15 The ability to predict and prevent clinical events, even when the patients are asymptomatic, will greatly streamline care and enhance outcomes.
The patients’ needs, their compliance, and the cost of the technologies will continue to be key drivers of not only the innovations, but also their use.
Disclosures: The authors have no conflicts of interest to report regarding the content herein.
See accompanying quiz on this topic at: https://www.eplabdigest.com/multimedia/expanding-pediatric-cardiac-monitoring-tool-box-experience-novel-ambulatory-cardiac-rhythm-monitoring-technologies
- Hauser R. Cost-effective ambulatory ECG monitoring. EP Lab Digest. 2020;5:22-24.
- Nguyen HH, Van Hare GF, Rudokas M, Bowman T, Silva JNA. SPEAR Trial: Smartphone Pediatric ElectrocARdiogram trial. PLoS ONE. 2015;10:e0136256.
- Macinnes M, Martin N, Fulton H, McLeod KA. Comparison of a smartphone-based ECG recording system with a standard cardiac event monitor in the investigation of palpitations in children. Arch Dis Child. 2019;104:43-47.
- Bolourchi M, Silver E, Muwanga D, Mendez E, Liberman L. Comparison of Holter with Zio patch electrocardiography monitoring in children. Am J Cardiol. 2019;125:767-771.
- Pradhan S, Robinson JA, Shivapour JK, Snyder CS. Ambulatory arrhythmia detection with ZIO XT patch in pediatric patients: a comparison of devices. Pediatr Cardiol. 2019;40:921-924.
- Rho R, Vossler M, Blancher S, Poole, J. Comparison of 2 ambulatory patch ECG monitors: the benefit of the P-wave and signal clarity. Am Heart J. 2018;203:109-117.
- Petrenas A, Marozas V, Jarusevicius G, Sornmo L. A modified Lewis ECG lead system for ambulatory monitoring of atrial arrhythmias. J Electrocardiol. 2015;48:157-163.
- Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18:241-247.
- Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75:2992-2993.
- Roy SK, Shah SU, Villa-Lopez E, et al. Comparison of electrocardiogram quality and clinic interpretations using prepositioned ECG electrodes and conventional individual electrodes. J Electrocardiol. 2020;59:126-133.
- Lin HJ, Lan YT, Silka MJ, et al. Home use of a compact, 12-lead ECG recording system for newborns. J Electrocardiol. 2019;53:89-94.
- Saarel EV, Granger S, Kaltman JR, et al. Electrocardiograms in healthy North American children in the digital age. Circ Arrhythm Electrophysiol. 2018;11:e005808.
- Rijbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA. New normal limits for the paediatric electrocardiogram. Eur Heart J. 2001;22:702-711.
- Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65-69.
- Attia ZI, Noseworthy P, Lopez-Jimenez F, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861-867.