The Comprehensive Approach to Atrial Fibrillation Management at Beth Israel Deaconess Medical Center
Atrial fibrillation (AF) is the most common arrhythmia in the United States, contributing to 454,000 hospitalizations annually.1 Roughly 4% of adults over age 60 carry the diagnosis, with a disease burden that is growing. Estimates show that 12.1 million Americans will be affected by 2030.2 Over the last several decades, the pathophysiology of AF has been better defined but is still not completely understood. Fortunately, the number of therapeutic options has grown exponentially.
Since its formation by Dr Mark Josephson, the Harvard-Thorndike Electrophysiology (EP) Institute and Arrhythmia Service at Beth Israel Deaconess Medical Center (BIDMC) has focused on a comprehensive approach to clinical evaluation as well as medical and procedural management of AF. In this article, we present our methods for delivering integrated, patient-centered AF care.
About the Program
The EP service is composed of 8 attending physicians, 6 EP fellows, 2 physician assistants, 3 outpatient nurse practitioners (NPs), 3 outpatient nurses, 1 device nurse, and 2 device technicians. The EP lab team consists of 18 nurses, 7 cross-trained cardiovascular and radiology techs, and 2 support staff. At BIDMC, procedures are performed in 3 dedicated EP labs; a fourth lab space is shared with interventional cardiology and used predominantly for device and left atrial appendage occlusion (LAAO) procedures. Other hybrid surgical cases for EP are performed in a separate hybrid operating room (OR).
A Holistic Approach to the AF Consult
To better serve our patients with AF, BIDMC developed one of the first dedicated AF clinics in the US as well as initiated the FRACTAL registry, a multicenter initiative to define practice patterns in patients with new-onset AF.3,4 This research has influenced an upstream approach to AF that is designed to provide early intervention and management. A cornerstone of this institutional approach to AF management includes a partnership with the emergency department (ED) to limit unnecessary hospital admissions and creation of a treatment algorithm to guide these decisions. Institution of this protocol demonstrated a reduced hospital admission rate for AF from 74% to 38%, which is likely even lower today.5
Another resource at BIDMC is the Cardiac Direct Access Unit (CDAc), which functions as a cardiac urgent care center. Patients can be seen same day for urgent outpatient appointments. We are able to obtain labs, imaging, and multidisciplinary consultations. If needed, patients can be monitored overnight in the CDAc inpatient observation unit. Patients have found the CDAc to be tremendously helpful in increasing their access to same-day cardiac care, while avoiding the stress and costs associated with evaluation in an ED.
Our EP service manages over 4000 inpatient and innumerable outpatient AF encounters per year. Attending physicians and NPs see outpatients 5 days a week, providing a vast array of device and arrhythmia management. Upon establishing care with our service, patients continue long-term follow-up through a personalized AF management approach that incorporates lifestyle and risk factor modification. Weight reduction strategies are presented as a parallel therapy for AF. Overweight patients are encouraged to lose at least 5% of their body weight before proceeding with ablation; referral to the BIDMC Weight Loss Program is often used to assist in this process. Given the link between AF and obstructive sleep apnea (OSA), significant emphasis is also placed on the diagnosis and management of sleep-disordered breathing. Untreated OSA can lead to increased afterload, left ventricular hypertrophy, and left atrial remodeling, all of which can complicate AF management. However, these sequelae can be combatted with effective therapies such as continuous positive airway pressure (CPAP). Patients who are compliant with their CPAP therapy have lower recurrences rates and better overall rhythm control of their AF compared to patients with untreated OSA.6 In addition, a novel implantable phrenic nerve stimulator is used for the treatment of central sleep apnea. If there is suspicion for sleep-disordered breathing, a home sleep study is ordered at the initial consult visit. For moderate to severe cases, we are able to expedite referral to a sleep specialist to more quickly improve a patient’s cardiac risk factor profile.
Regarding symptomatic management of AF, we utilize a shared decision-making model to proceed with either a rate or rhythm control strategy after discussion of the risks and benefits. Catheter ablation and antiarrhythmic drugs (AADs) are used to achieve rhythm control; however, we commonly recommend catheter ablation as a first-line option due to the improved safety and efficacy outcomes associated with early pulmonary vein isolation (PVI).7
If rhythm control via AADs is desired, we offer all FDA-approved options. We safely begin certain AADs (eg, amiodarone, sotalol, and class Ia and Ic agents) in the outpatient setting to minimize hospital admissions. Patients must be in sinus rhythm at initiation and are prescribed an event recorder to send once- or twice-daily transmissions that are reviewed by an electrophysiologist for brady- and tachyarrhythmias as well as proarrhythmic effects such as QTc prolongation.8
Regarding anticoagulation, direct oral anticoagulants are favored and prescribed as a first-line option. However, there are patients for whom lifelong anticoagulation is undesirable or contraindicated. Through pilot studies, we have demonstrated that a tailored approach to anticoagulation based on device-detected burden of AF is feasible in low CHA2DS2-VASc score patients. We are hoping to help coordinate definitive large-scale studies to validate the safety of this tailored anticoagulation approach in the future.9
For those patients in whom lifelong anticoagulation is contraindicated, we have partnered with our structural heart colleagues to offer LAAO as an alternative. Through a scheduled telemedicine visit, patients are introduced to the team and evaluated as a potential candidate for percutaneous or surgical closure. Our transcatheter LAAO cases are performed jointly by an EP and a structural heart operator and followed postoperatively by our LAAO coordinator.
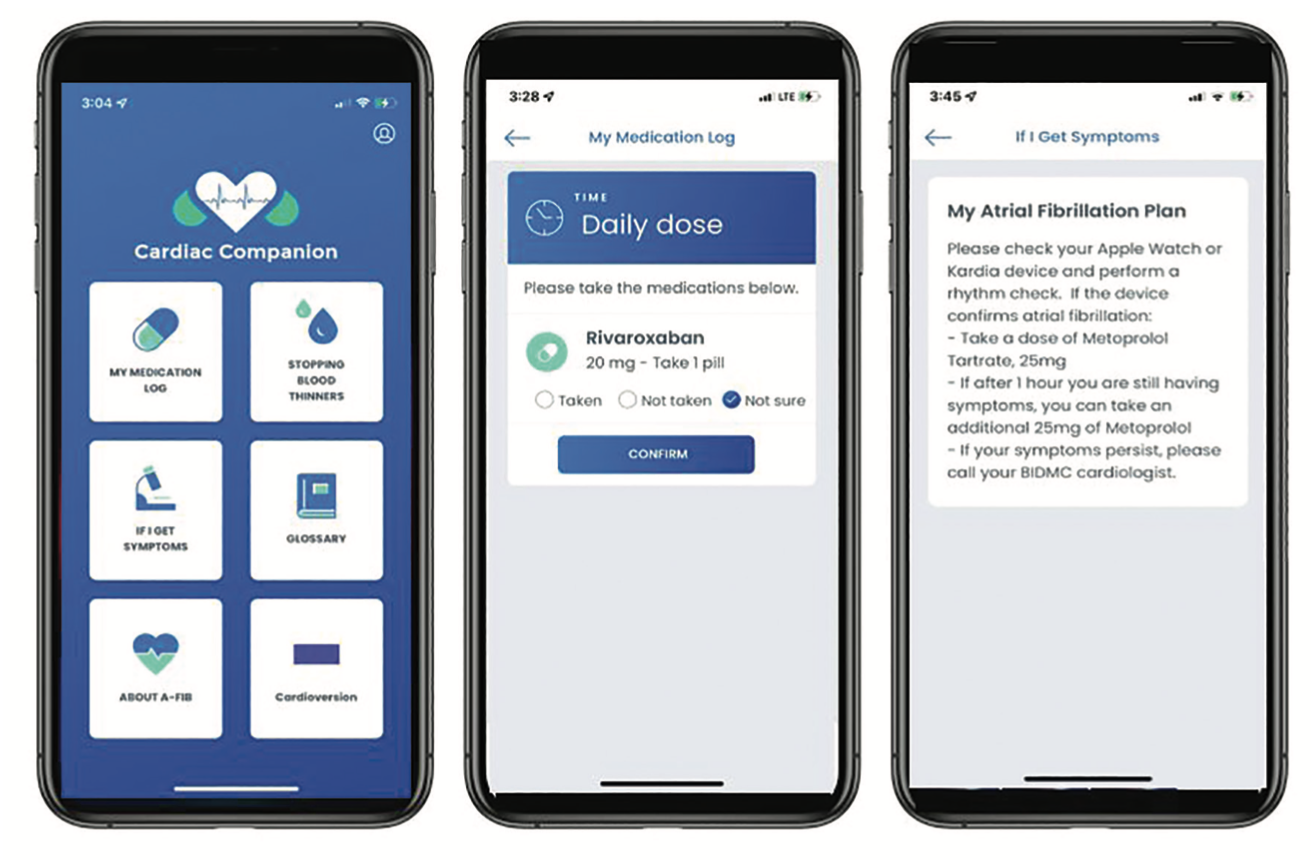
One of the most exciting new developments at BIDMC is the development of an arrhythmia smartphone app, Cardiac Companion, available on iPhone and Android devices (Figure 1). Currently, the app is being trialed with a small patient cohort before officially launching to all our cardiology patients. The app was designed to offer patient-centered clinical information as well as medication management pathways. General AF education materials are available for all patients to review at their leisure. Providers are also able to customize treatment plans and tailor instructions to each patient. Patients with AF will be provided with a personalized “My AF Plan” that is developed by their provider and that clearly states an action plan for symptomatic recurrences (ie, rhythm check with personal wearable device, supplemental doses of beta-blockers for symptom management). “My AF Plan” will also assist with medication compliance by sending daily reminders. Our hope is that this will reduce the risk of dangerous outcomes such as thromboembolic events. We anticipate that the app will be available to all our patients in Spring 2022.
AF Ablation: Procedural Experience
AF ablation has become the most common procedure in our EP lab. As our experience has grown, we have standardized our protocols. All AF ablation procedures are performed on uninterrupted anticoagulation and under general anesthesia with high frequency jet ventilation (HFJV). Use of HFJV allows for minimal respiratory motion and improved catheter stability, potentially improving procedural efficacy.
In the past, bilateral femoral venous access was commonly used to accommodate a dual transseptal approach. However, we are now using only a unilateral access approach.
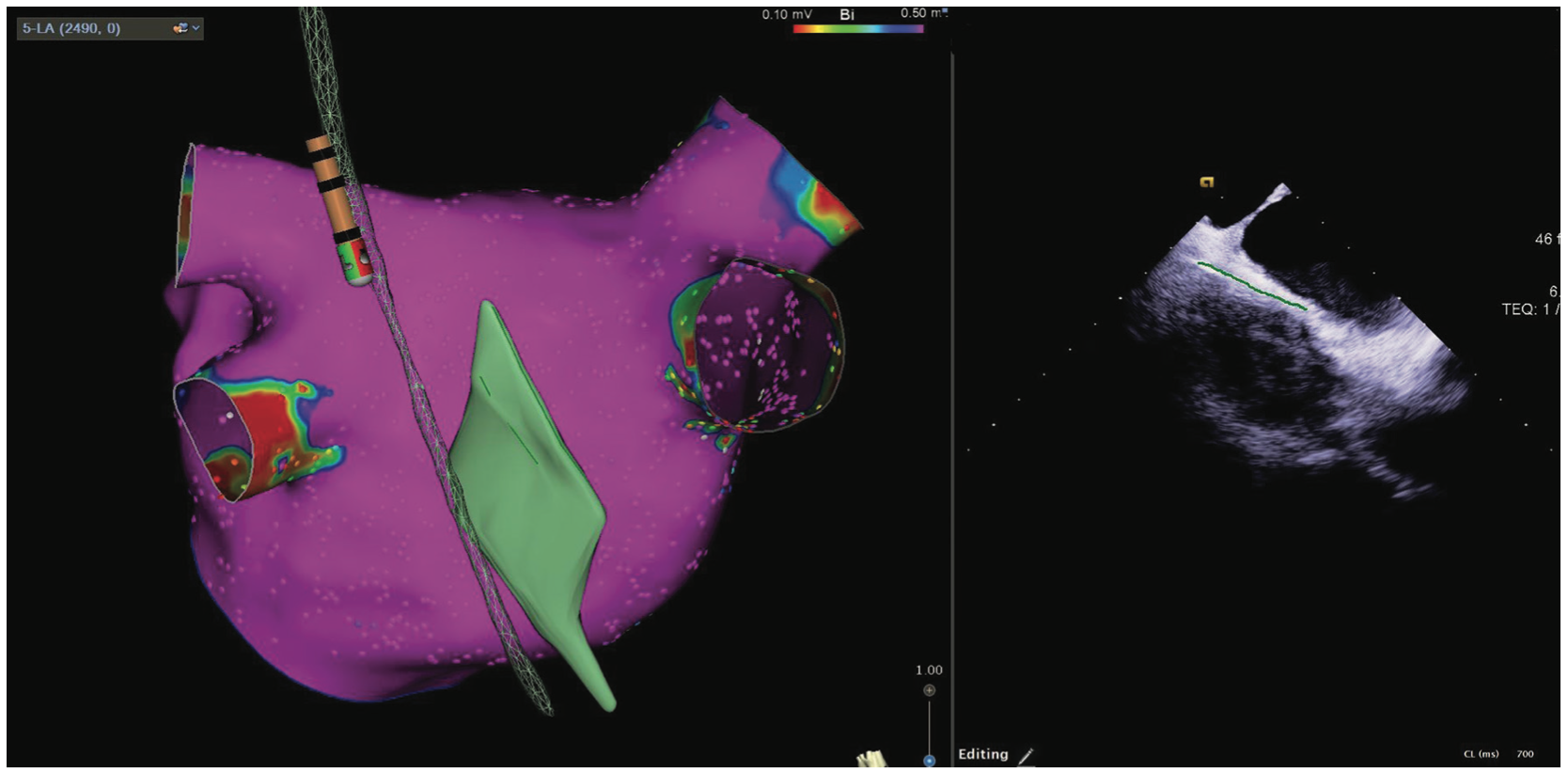
In an effort to avoid esophageal injury, our lab has been vigilant in reducing the amount of radiofrequency ablation time adjacent to the esophagus. In all left atrial ablations, the esophageal location relative to the posterior wall of the left atrium is defined. We accomplish this in 2 ways (Figure 2). The first approach is to directly integrate and visualize esophageal geometry in an electroanatomical mapping (EAM) system using a combined esophageal temperature probe and mapping catheter. This allows for positioning as well as esophageal temperature monitoring without fluoroscopy. Another method for esophageal protection is to measure and outline the width of the esophagus via integrated intracardiac echocardiography (ICE) and EAM. The Esophastar (Biosense Webster, Inc, a Johnson & Johnson company) catheter is nondeflectable and can show position in the vertical plane. Through integrated ICE and EAM, we are able to see anatomical variations in depth and width as well as the overall position of the esophagus, and incorporate this into the mapping system. This is particularly important when planning our ablation strategy across the posterior wall.
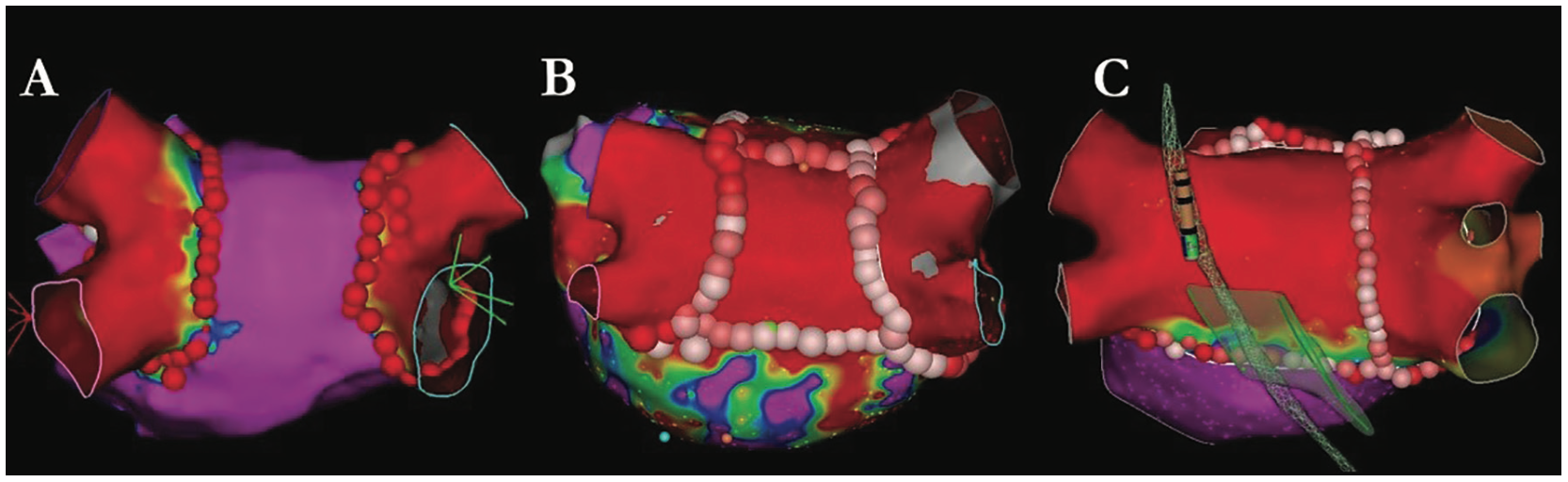
The ablation strategy is customized to the patient’s clinical characteristics of AF as well as individual left atrial anatomy. In paroxysmal AF, an isolated PVI lesion set is used in first-time patients as an initial strategy. In some paroxysmal cases, an EP study is performed at the end of the ablation to evaluate for inducible AF or atrial flutters. In certain cases where AF remains inducible, we will complete a posterior wall isolation if this has not already been performed. In persistent AF, a PVI lesion set is combined with posterior wall isolation as an initial approach. Figure 3 illustrates our PVI strategy for paroxysmal AF, persistent AF, and persistent AF with the esophageal location overriding the left PVs.
As our experience with PVI increases, our use of ICE and a fluoroless workflow has also expanded. Nearly 60% of all AF ablation cases are performed without radiation at this time. When fluoroscopy is used, the average fluoroscopy time is 5.57 minutes and the average radiation exposure is 12.4 mGy.
At the conclusion of the ablation, a single figure-of-8 suture is placed while the patient is still under anesthesia. This helps achieve hemostasis without the typical long-duration manual pressure that was required in the past. It has also greatly improved patient comfort upon awakening from anesthesia.
In cases where durable rhythm control is not possible, we follow an “ablate and pace” strategy as last-line therapy, focusing on cardiac resynchronization therapy or physiologic pacing (either His bundle or left bundle branch pacing) followed by atrioventricular junction ablation. In the appropriate patient, we have found this improves quality of life while minimizing the left ventricular dysfunction associated with 100% traditional right ventricular pacing.
Postprocedural Care
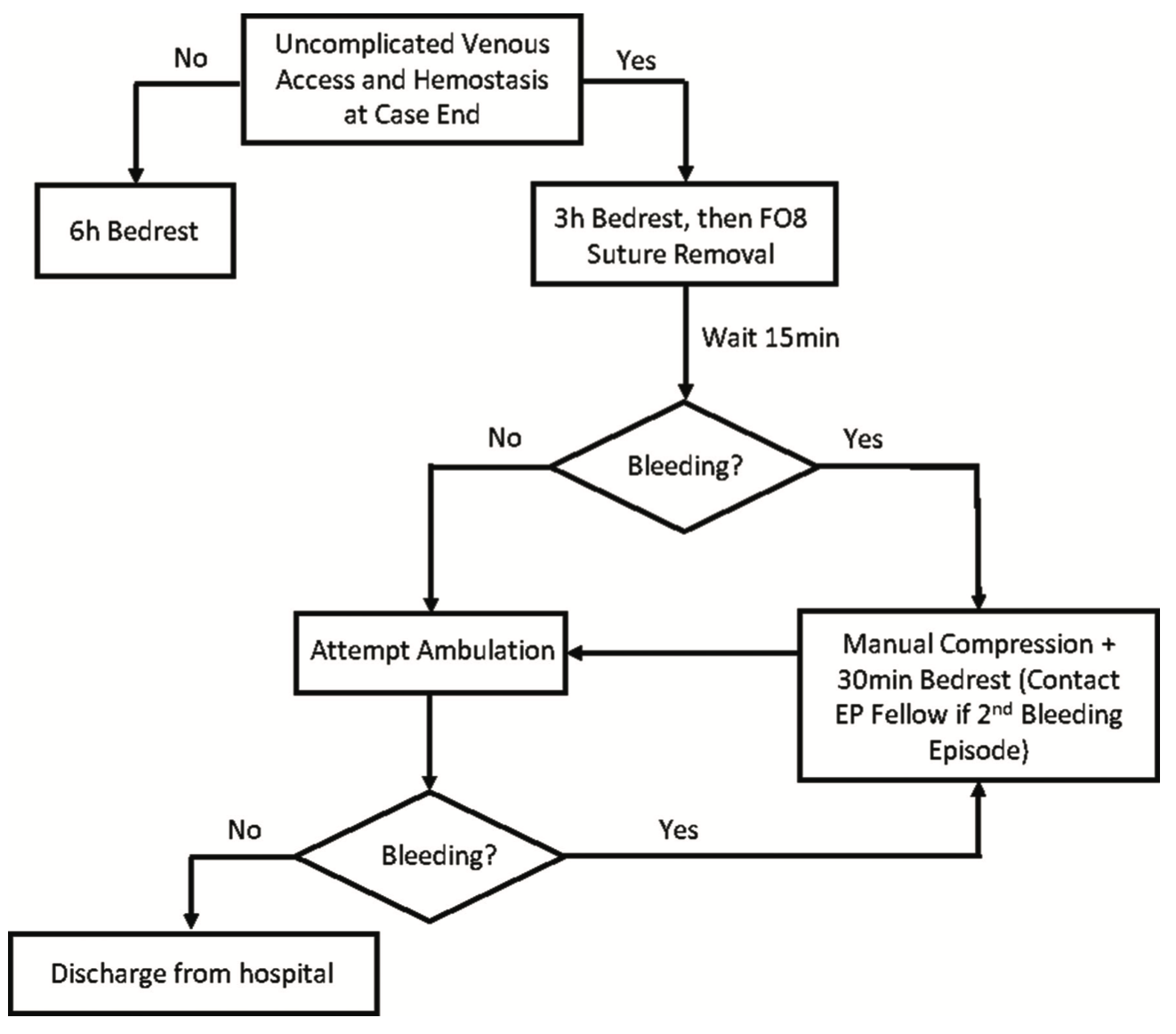
The COVID-19 pandemic offered an opportunity to review our procedural workflow and consider creative solutions to the bed crunch felt everywhere. Previously, our PVI patients were admitted overnight and discharged the next day. When this was no longer sustainable, we developed a same-day discharge protocol that has been very successful (Figure 4).
Utilizing this approach, our same-day discharge rate post-AF ablation grew from less than 5% in 2019 to over 85% in 2021. With this dramatic increase in same-day discharge, we were initially concerned that we would also see an increase in periprocedural complications. Happily, our data have shown no significant increases in readmission or vascular access complication rates. By utilizing a figure-of-8 stitch at the access site in conjunction with limited manual pressure, many patients are able to safely ambulate after 3 hours of bed rest, with no increase in bleeding complications from access sites.
A 30-day prescription for a proton pump inhibitor is routinely provided for esophageal protection if the patient was not previously prescribed one. If a recently discharged patient has a concern related to their procedure, we provide a dedicated phone number called the “HeartLine.” This is staffed 24 hours a day, 7 days a week. During the day, coverage is provided on a rotating basis by our advanced practice providers across the cardiovascular service line; an on-call physician provides coverage overnight. If the provider feels that the patient requires in-person evaluation, same-day referral to the CDAc can be easily initiated. Overall, the vast majority of our patients do not require immediate care postablation and are ultimately seen in the EP clinic 6-12 weeks postdischarge.
New Directions
Throughout the last decade, we have seen an exponential increase in therapeutic options for patients with AF. Ablation therapies that are safer and more efficacious continue to be developed. The Harvard-Thorndike EP Institute and Arrhythmia Service at BIDMC has a rich history of advancing the field through clinical research, including the mechanistic understanding of arrhythmias and novel device development. Today, we are involved in multiple national and international trials, including 2 clinical trials evaluating novel medical therapies (InRhythm, InCarda Therapeutics; and HSY244, Novartis) for outpatient pharmacologic cardioversion, the CATALYST trial evaluating the safety and effectiveness of a novel LAA occluder compared to novel oral anticoagulation therapy, and the ADVENT trial evaluating a novel energy source for AF ablation.
Summary
As the US population ages, the number of patients with AF will continue to grow. Our approach to AF treatment and management recognizes the many factors associated with successful maintenance of sinus rhythm. BIDMC will continue to embrace future challenges as opportunities, and remain at the forefront of integrated, comprehensive, patient-centered AF care.
Disclosures: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Zimetbaum reports consulting fees from Abbott, Farapulse (CEC Committee), and Medtronic. The other authors have no conflicts of interest to report regarding the content herein.
References
1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142-1147. doi:10.1016/j.amjcard.2013.05.063
3. Reynolds MR, Lavelle T, Essebag V, et al. Influence of age, gender, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152(6):1097-1103. doi:10.1016/j.ahj.2006.08.011
4. Reynolds MR, Essebag V, Zimetbaum P, et al. Healthcare resource utilization and costs associated with recurrent episodes of atrial fibrillation: the FRACTAL registry. J Cardiovasc Electrophysiol. 2007;18(6):628-633. doi:10.1111/j.1540-8167.2007.00819.x
5. Zimetbaum P, Reynolds MR, Ho KKL, et al. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92(6):677-681. doi:10.1016/s0002-9149(03)00821-x
6. Marulanda-Londoño E, Chaturvedi S. The interplay between obstructive sleep apnea and atrial fibrillation. Front Neurol. 2017;8:668. doi: 10.3389/fneur.2017.00668
7. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444. doi:10.1016/j.hrthm.2017.05.012
8. Hauser TH, Pinto DS, Josephson ME, Zimetbaum P. Safety and feasibility of a clinical pathway for the outpatient initiation of antiarrhythmic medications in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2003;91(12):1437-1441. doi:10.1016/s0002-9149(03)00395-3
9. Waks JW, Passman RS, Matos J, et al. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dual-chamber pacemakers and implantable cardioverter-defibrillators: results from the Tailored Anticoagulation for Non-Continuous Atrial Fibrillation (TACTIC-AF) pilot study. Heart Rhythm. 2018;15(11):1601-1607. doi:10.1016/j.hrthm.2018.06.027











