Computed Tomography and Late-Iodine Enhancement in Guiding Substrate Mapping and Ablation of Ventricular Tachycardias
Identification of ventricular tachycardia (VT) substrate for successful catheter ablation may be challenging. In the modern era of catheter ablation, electroanatomic mapping (EAM) is used in conjunction with conventional electrophysiologic methods to characterize myocardial substrate in tachyarrhythmias. However, EAM alone has limitations in delineating complex and transmural scar. Therefore, integration of advanced cardiac imaging is a strategy to improve substrate characterization.1
Echocardiography may be insensitive in defining the endocardial border, wall motion, thinning, and scar, especially in severely depressed left ventricular function.2 Magnetic resonance imaging with late-gadolinium enhancement provides additional information on myocardial scar localization but may be problematic due to cost and in patients with cardiac implantable electronic devices.3-6
A novel method for assessment of wall thinning and scar localization using late-iodine enhancement on computed tomography (CT) can be helpful in identifying VT substrate.7-9 We present a VT ablation case where CT imaging was fused with high-density EAM to delineate VT circuitry.
Case Presentation
A 66-year-old male was referred to our center for management of recurrent VT. He had a history of acute myocardial infarction and had undergone coronary bypass surgery several years prior. He subsequently developed ischemic cardiomyopathy and heart failure with a reduced ejection fraction of 15-20% despite optimal medical therapy, necessitating the implantation of a dual-chamber implantable cardioverter-defibrillator for primary prevention of sudden cardiac death. He later developed VT and was treated by his primary cardiologist. He experienced breakthrough VT on oral amiodarone therapy and received frequent therapies from his defibrillator.
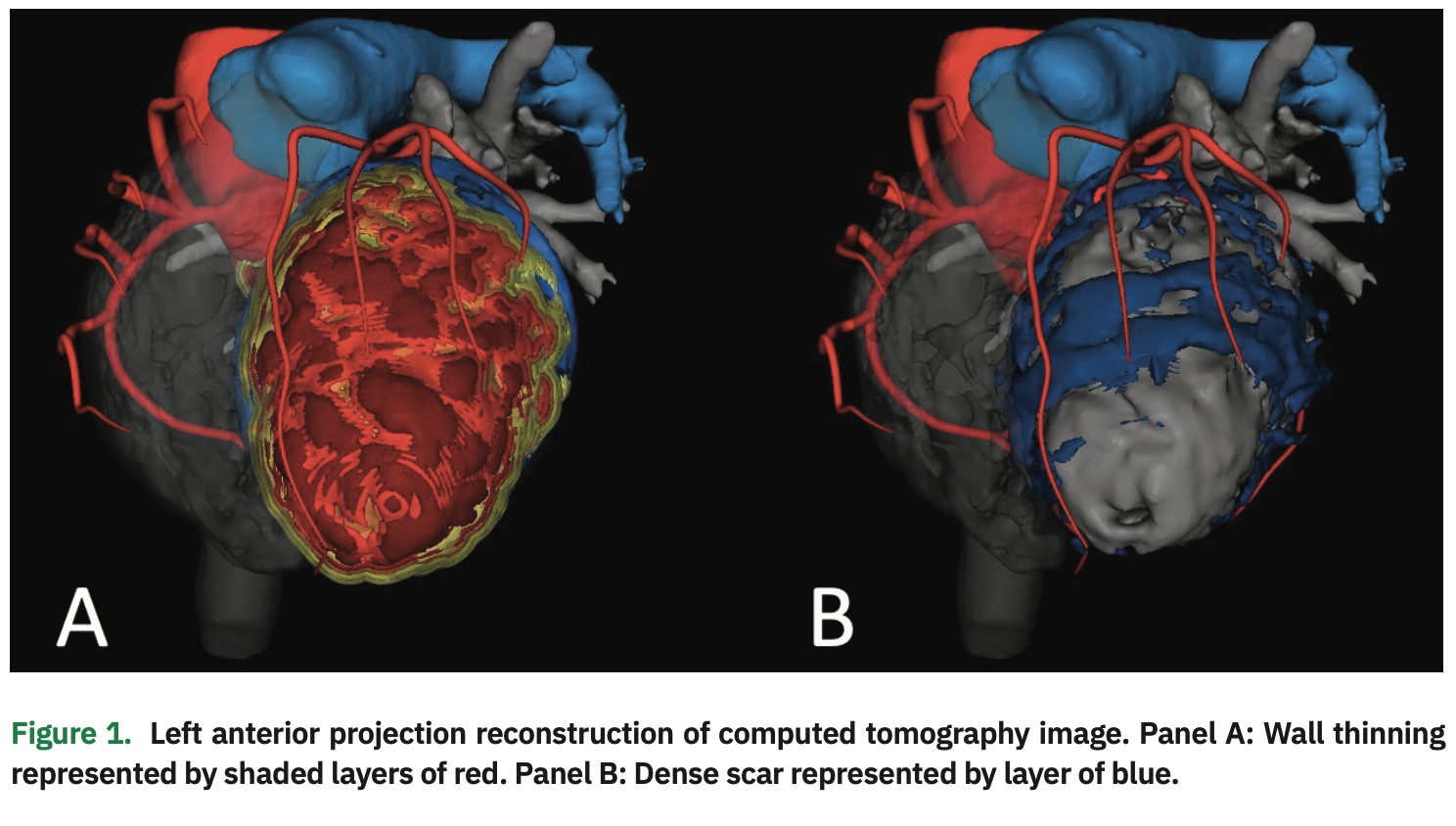
Prior to VT catheter ablation, the patient underwent advanced imaging with CT to assess regions of wall thinning and dense myocardial scar. CT imaging revealed a large area of wall thinning extending from the base to apex of the left ventricular anterior and anterolateral walls. However, comparison of isolated dense scar revealed a potential isthmus located in the mid-anterior segment (Figure 1).
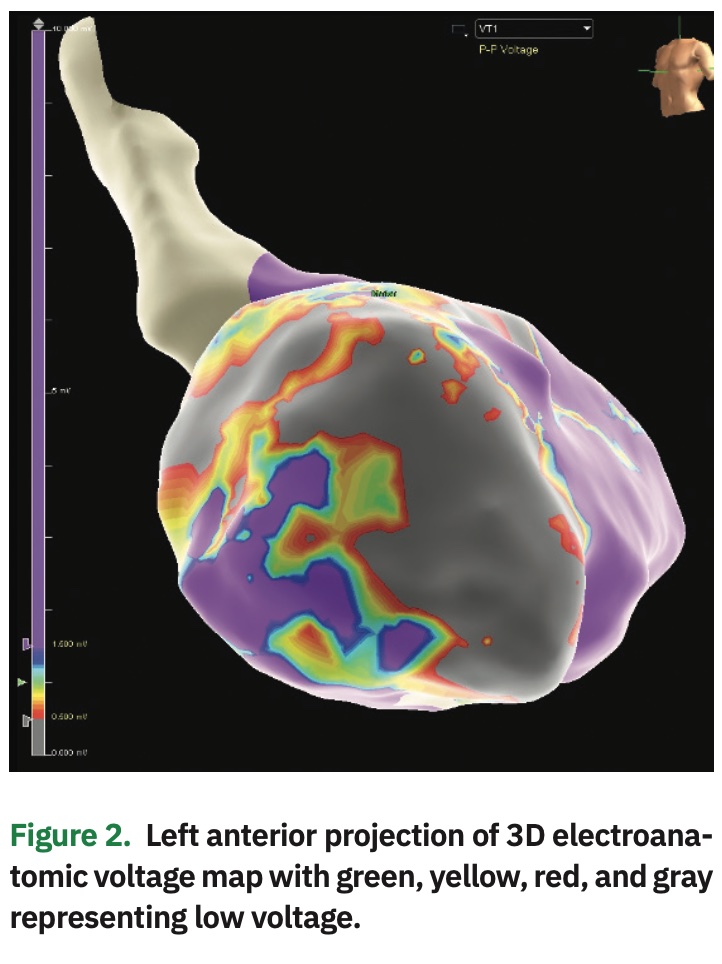
The patient was taken to the cardiac electrophysiology laboratory in a post-absorptive state, and femoral vascular access was obtained in standard fashion. Multipolar catheters were inserted into the coronary sinus and right ventricular apex for recording and pacing. Next, an endocardial map of the left ventricle was created via retrograde aortic and transseptal approach using the Advisor HD Grid SE Mapping Catheter and EnSite Precision Cardiac Mapping System (both Abbott). Voltage map demonstrated scar along the anterior wall, correlating with wall thinning noted on CT (Figure 2).
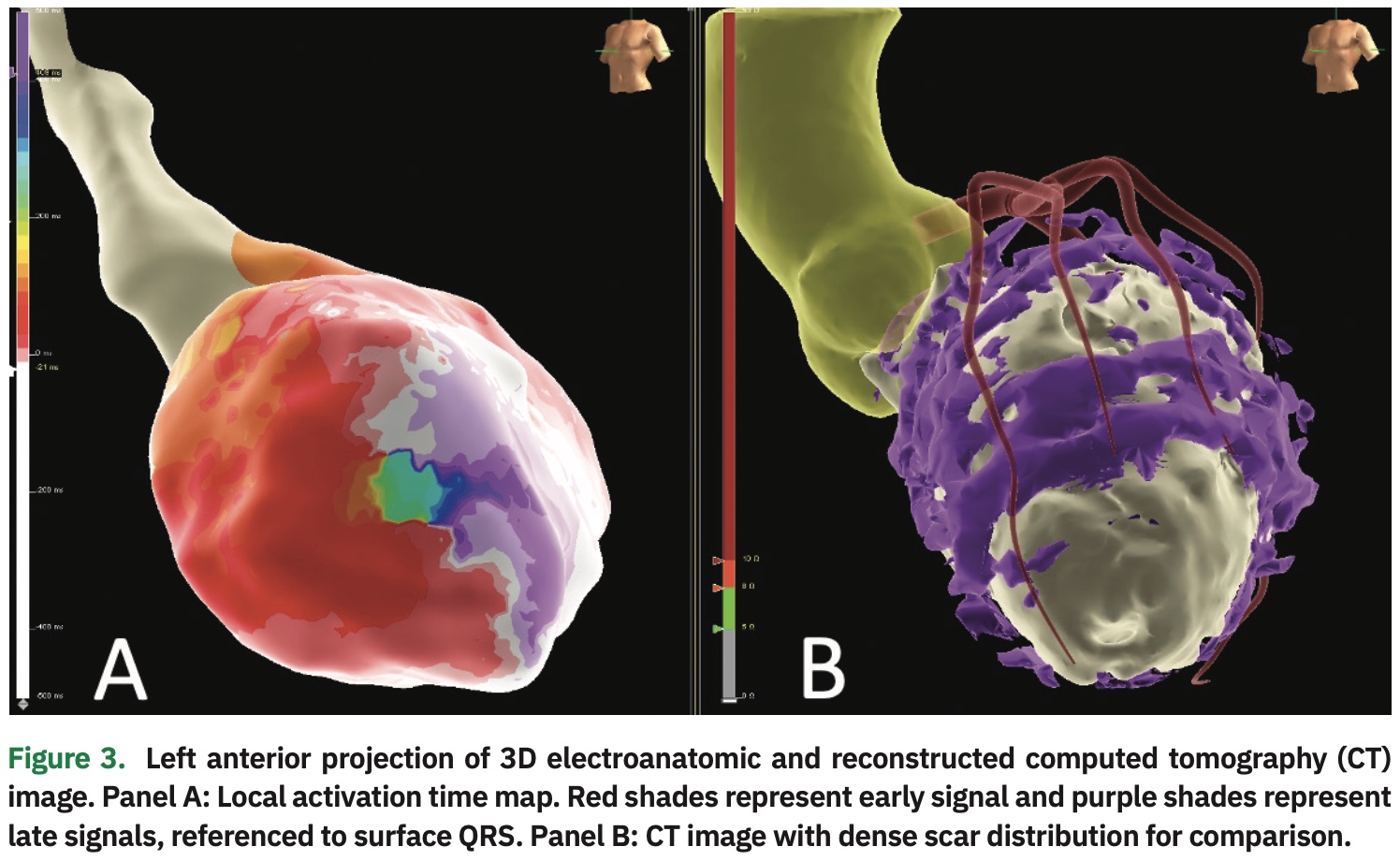
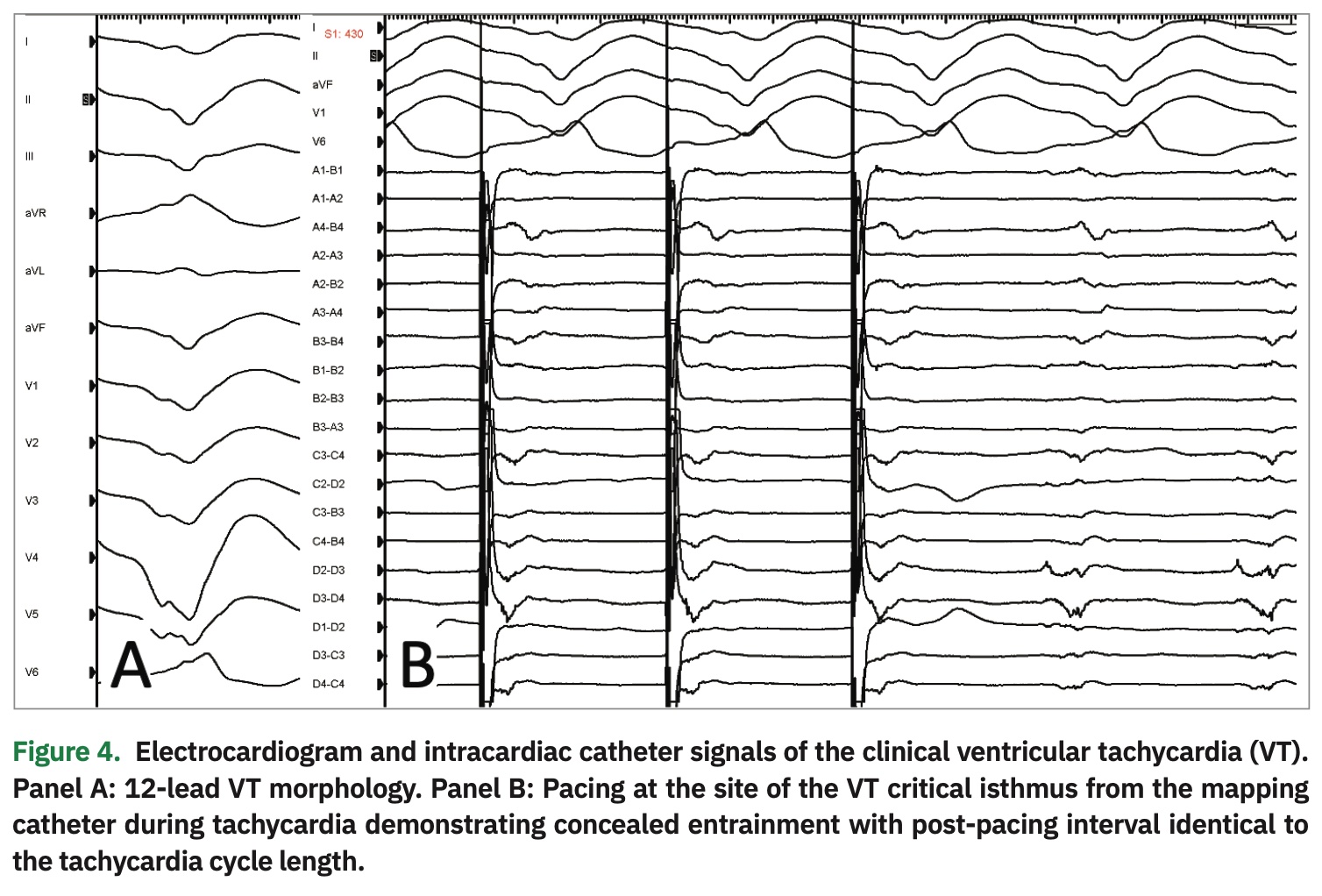
With catheter manipulation, the clinical VT was easily inducible. Hemodynamic support was achieved with vasopressors, and activation mapping of the left ventricle was performed. The local activation time referenced to the surface QRS is shown in Figure 3. The signal wavefront propagation sequence exhibited an easily identifiable critical isthmus for the clinical VT (Video 1). To confirm this finding via conventional methods for mapping of re-entrant tachycardias, the mapping catheter was positioned on the isthmus and VT was induced with programmed extra-stimulation. Next, overdrive pacing demonstrated concealed entrainment with a short stim-to-QRS and post-pacing interval identical to the tachycardia cycle length (Figure 4).
Catheter ablation with radiofrequency energy was performed on the critical isthmus and adjacent myocardium. Successful lesion creation was confirmed with high output pacing to demonstrate non-capture or exit block within the isolated region. Repeat programmed extra-stimulation under the influence of isoproterenol infusion failed to induce tachycardia, successfully concluding the procedure. The patient is arrhythmia-free and off antiarrhythmic medications at 6-month follow-up.
Discussion
Contemporary multidetector CT scanners are capable of obtaining high-resolution images for the reconstruction of complex anatomic structures. Three-dimensional reconstruction of dual-energy CT images accompanied by late-iodine enhancement, similar to late-gadolinium enhancement, can provide detailed information regarding myocardial substrate architecture.7-9 This technology, coupled with high-density electroanatomic arrhythmia mapping, results in improved delineation of tachycardia circuitry for targeted catheter ablation.7-9
The kinetics of myocardial wash-in and washout of gadolinium and iodine contrast agents are similar.8 Although CT late-iodine enhancement protocols vary, an approximate injection of 0.6 grams of iodine per kilogram of body weight, followed by imaging acquisition 7-10 minutes after contrast injection is necessary for image acquisition.7 Dual-energy CT demonstrates improvement
in contrast-to-noise ratios and beam-hardening artifacts that have proved similar performance between late-iodine compared to late-gadolinium enhancement in cardiac magnetic resonance imaging.11 In comparison to late-gadolinium enhancement, CT late-iodine enhancement was excellent when evaluated by the most experienced readers. Moreover, the agreement between late-iodine and late-gadolinium in evaluating the presence or absence of scar was 100% in ischemic substrate versus 75 to 90% in non-ischemic substrate.12
Furthermore, wall thinning alone imaged either by CT, magnetic resonance, or echocardiography as shown in Figure 1 could be misleading and insufficient. The addition of late-iodine enhancement data revealed distribution of scar for improved demarcation of the VT critical isthmus. In this case, scar distribution correlated highly with the VT critical isthmus as demonstrated by the signal wavefront propagation map. This enhanced method of scar characterization resulted in successful ablation.
Disclosures: The authors have no conflicts of interest to report regarding the content herein.
Video 1:
- Dinov B, Schönbauer R, Hordynska-Wojdyla A, et al. Long-term efficacy of single procedure remote magnetic catheter navigation for ablation of ischemic ventricular tachycardia: a retrospective study. J Cardiovasc Electrophysiol. 2012;23:499-505.
- Spencer KT, Bednarz J, Mor-Avi V, et al. The role of echocardiographic harmonic imaging and contrast enhancement for improvement of endocardial border delineation. J Am Soc Echocardiogr. 2000;13:131-138.
- Esposito A, Gallone G, Palmisano A, Marchitelli L, Catapano F, Francone M. The current landscape of imaging recommendations in cardiovascular clinical guidelines: toward an imaging-guided precision medicine. Radiol Med. 2020:125:1013-1023.
- Shulman RM, Hunt B. Cardiac implanted electronic devices and MRI safety in 2018 – the state of play. Eur Radiol. 2018;28:4062-4065.
- Cronin EM, Mahon N, Wilkoff BL. MRI in patients with cardiac implantable electronic devices. Expert Rev Med Devices. 2012;9:139-146.
- Vitrella G, Faganello G, Morea G, et al. Role of cardiac imaging: cardiac magnetic resonance and cardiac computed tomography. 2019 May 18. In: Sinagra G, Merlo M, Pinamonti B, (eds). Dilated Cardiomyopathy: From Genetics to Clinical Management. Cham (CH): Springer; 2019. Chapter 8.
- Esposito A, Palmisano A, Antunes S, et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: relationship between CT-segmented scar and electro-anatomic mapping. JACC Cardiovasc Imaging. 2016;9:822-832.
- Yamashita S, Sacher F, Mahida S, et al. Imaging integration to guide catheter ablation in scar-related ventricular tachycardia. J Cardiovasc Electrophysiol. 2016;27:699-708.
- Cochet H, Komatsu Y, Sacher F, et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: a pilot study. J Cardiovasc Electrophysiol. 2013;24:419-426.
- Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823-833.
- Ohta Y, Kitao S, Yunaga H. Myocardial delayed enhancement CT for the evaluation of heart failure: comparison to MRI. Radiology. 2018;288:682-691.
- Palmisano A, Vignale D, Benedetti G, et al. Late iodine enhancement cardiac computed tomography for detection of myocardial scars: impact of experience in the clinical practice. Radiol Med. 2020;125:128-136.