Catheter Ablation for Atrial Fibrillation Without X-Rays: The Zero Fluoro Technique
Introduction
Catheter ablation is currently the most effective treatment for atrial fibrillation (AF).1,2 It is widely performed in various centers around the world given the increasing prevalence of AF in the population and the modest response to antiarrhythmic medications.
As with most percutaneous cardiac procedures, fluoroscopy has been a fundamental imaging modality to manipulate catheters in the vascular and cardiac chambers. However, ionizing radiation has multiple potential deleterious effects for both patients and the health care team.3-6 These effects are cumulative and add to an already frequent exposure due to high usage in diagnostic and therapeutic imaging modalities.7 In that regard, the ALARA (As Low As Reasonably Achievable) principle has been proposed to limit radiation use to the minimum needed to meet the objective.3
In that regard, the ALARA (As Low As Reasonably Achievable) principle has been proposed to limit radiation use to the minimum needed to meet the objective.3 In recent years, several efforts have succeeded in decreasing radiation exposure during AF ablation procedures, including reducing fluoroscopy times and doses,8,9 utilizing better shielding, and most importantly, using non-fluoroscopic imaging modalities such as 3D electroanatomical systems (EA) and intracardiac echocardiography (ICE).
In fact, use of non-fluoroscopic tools in the EP lab has increased so much over the years that it is now possible to guide the entire ablation procedure completely without the use of x-ray.10 First reported about 10 years ago,11,12 the zero fluoro technique has gained popularity in the EP community, as it has shown to be as safe and effective as procedures guided by fluoroscopy.13-15 It is our perception that, after a learning curve, in most instances the visualization and manipulation are indeed more precise than with fluoroscopy, without any blind parts.
The case presented here shows how multiple targets in both atria can be precisely reached and successfully ablated, even in the presence of previously implanted pacemaker leads.
Case Description
A 70-year-old male patient was referred for AF ablation due to recurrent episodes of AF and atrial flutter not responsive to sotalol and amiodarone. His medical history is significant for coronary artery bypass grafting (CABG) 8 years before, and a DDDR pacemaker implanted 3 years ago due to complete heart block. The patient has since then become pacemaker dependent, with 100% ventricular paced rhythm. Left ventricular function is normal, and the left atrium is only mildly dilated.
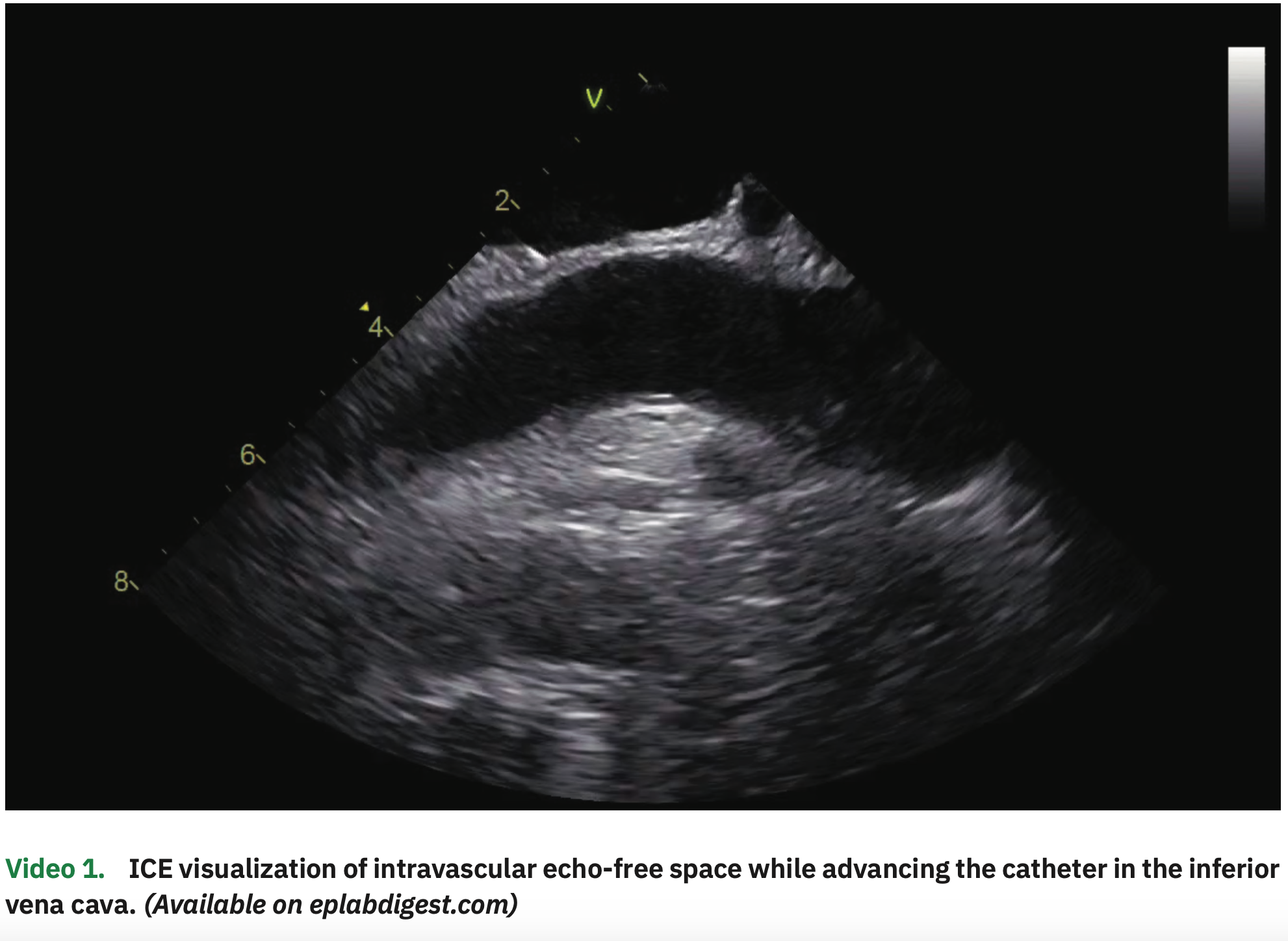
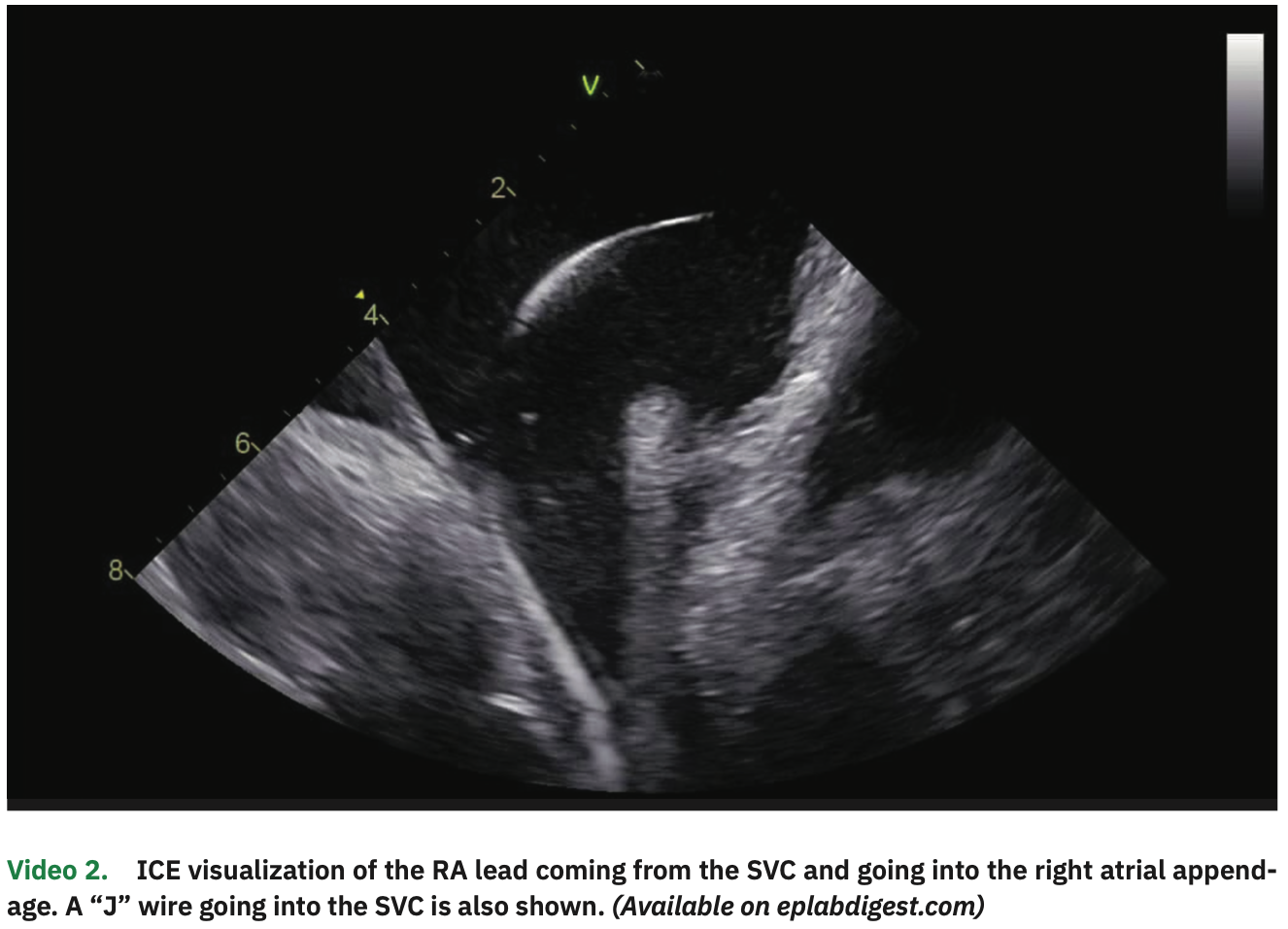
The procedure was performed under general anesthesia and on uninterrupted apixaban (last dose in the evening before the early morning procedure). The patient was in paced rhythm both in the atrial and ventricular channels. Venous access was obtained in the right and left femorals as well as in the right internal jugular vein, all guided by ultrasound. From the left femoral vein, an ICE catheter was advanced to the right atrium (RA), guided by visualization of echo-free spaces in the vascular system (Video 1). The pacemaker leads were clearly visualized in the RV septum and right atrial appendage (Figure 1 and Video 2). A multipolar esophageal catheter was placed and its position guided by ICE visualization.
Two guidewires were inserted from the right femoral vein and advanced to the superior vena cava (SVC), with adequate positioning visualized on ICE. Two long transseptal sheaths (fixed curve and deflectable) were advanced over the wire to the SVC. Also, a duodecapolar catheter was advanced from the right jugular vein, and entry into the RA was visualized on ICE.
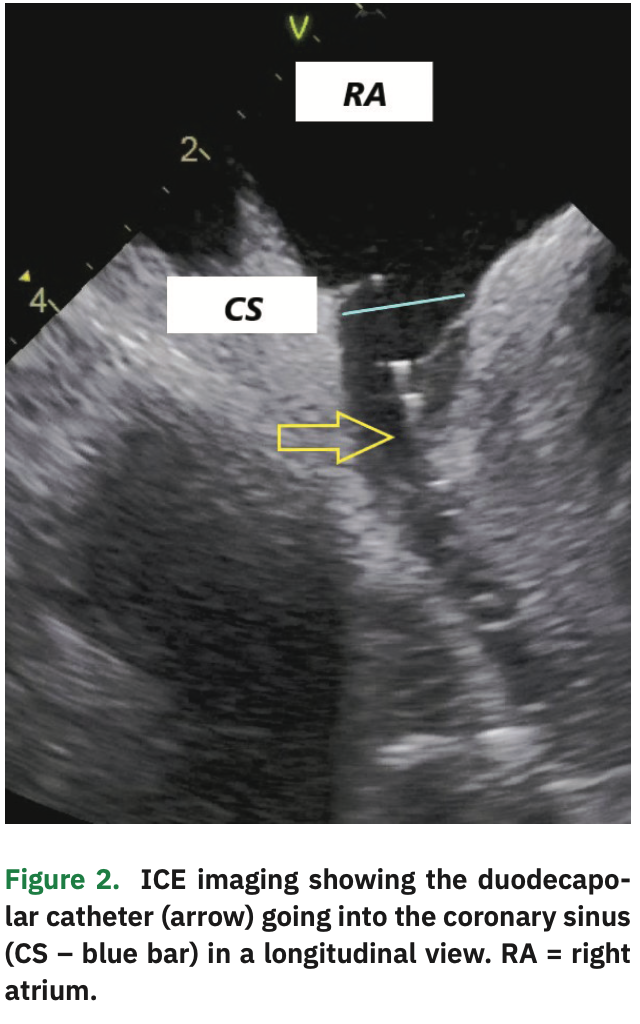
An irrigated 3.5 mm ablation catheter with contact force sensor was advanced under EA visualization and a limited right atrial map constructed, mostly to create a matrix (allowing other catheters to be visualized in EA maps) and to delineate the septum and CS anatomy. The CS was cannulated with the duodecapolar catheter under EA and ICE visualization (Figure 2). The distal 10 poles were in the CS, with the proximal poles in the RA.
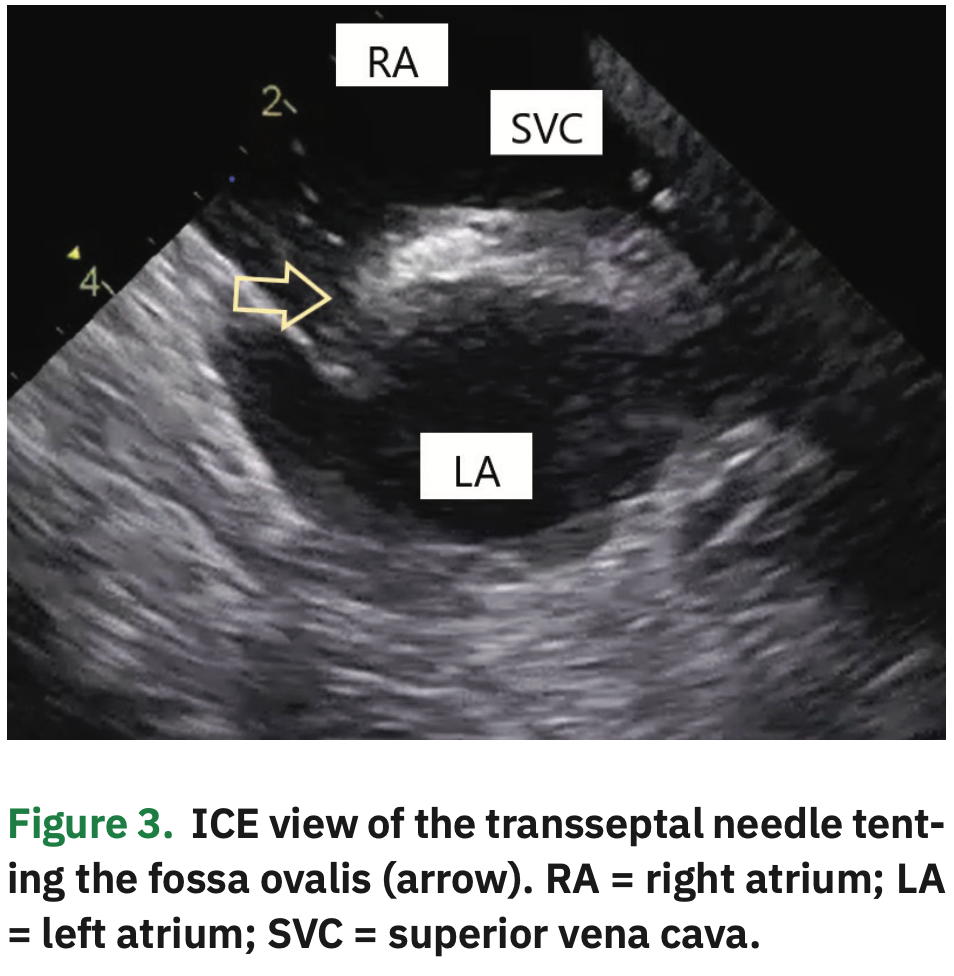
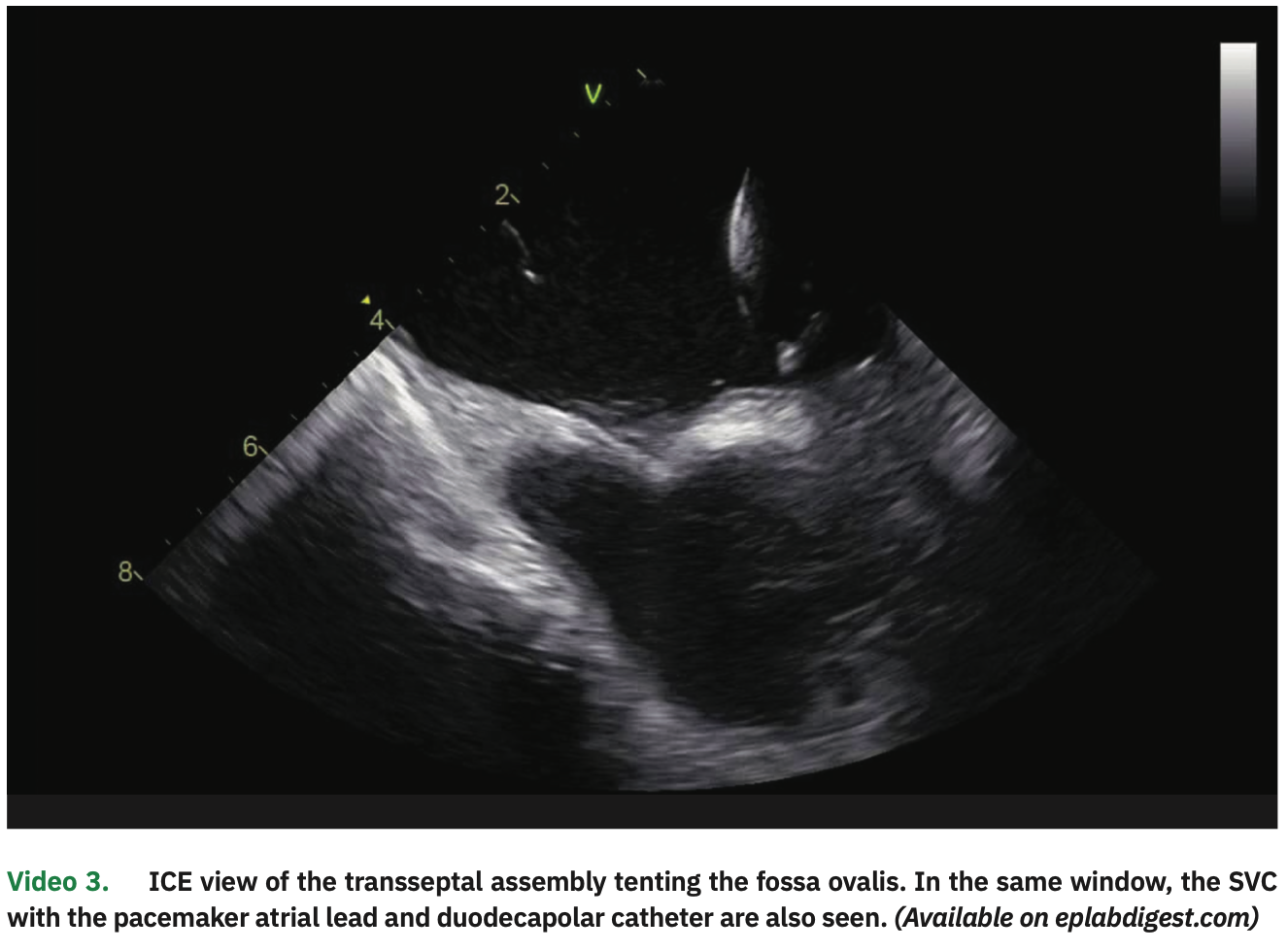
Two transseptal punctures were separately performed under ICE visualization, coming down to the septum from the SVC (Figure 3 and Video 3). After each septal perforation, a guidewire was advanced to the left superior pulmonary vein (LSPV), thus allowing for safe over the wire sheath positioning in the left atrial (LA) cavity. The ablation catheter and a multipolar spline catheter were then positioned in the PVs. All these steps were clearly visualized on ICE.
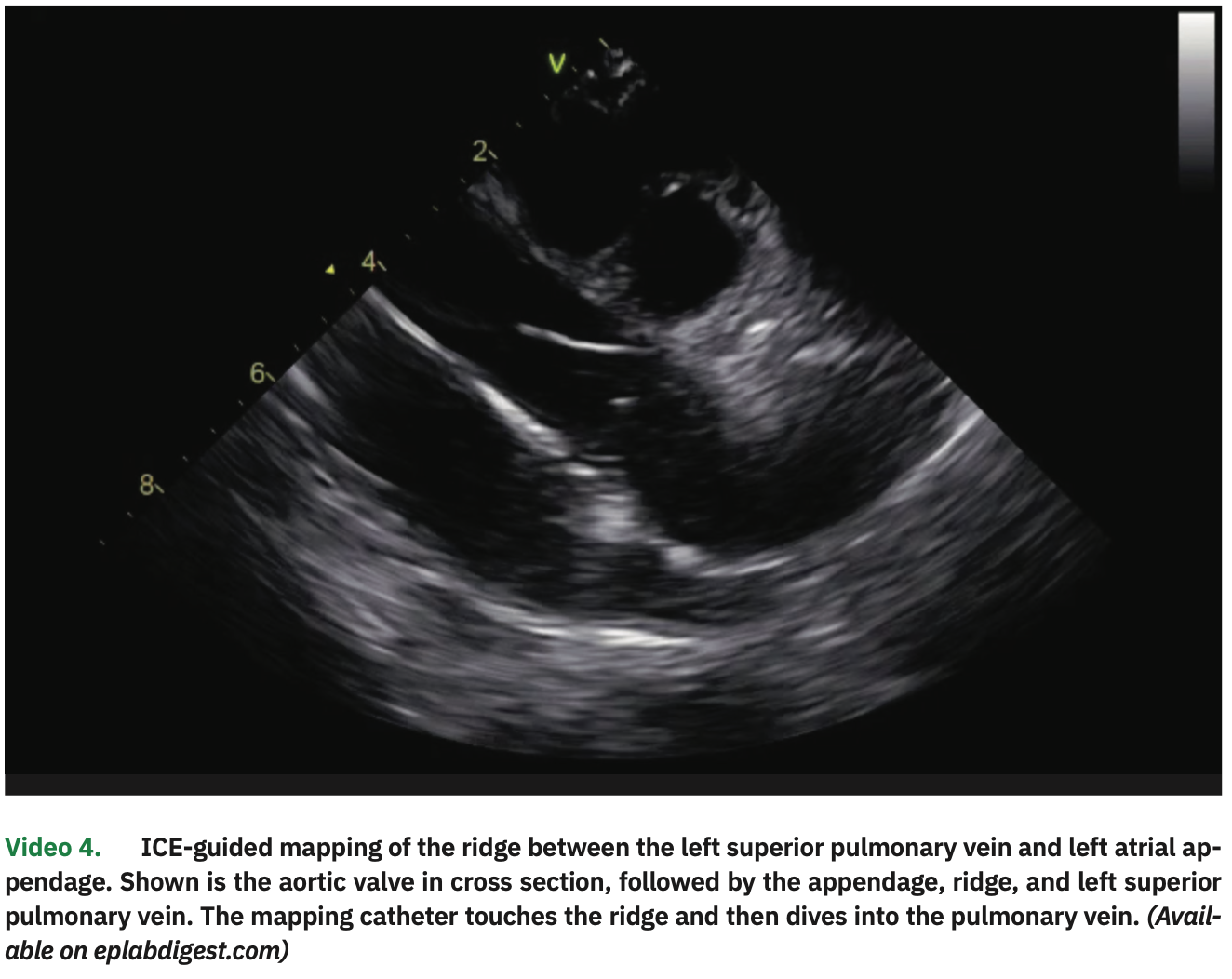
The LA and PV anatomies were reconstructed with a high-density map using the multipolar catheter. In particular, the ridge between the LSPV and left atrial appendage was visualized on ICE (with the catheter placed in the right ventricle; Video 4) and its position was manually annotated in the EA map. After calibrating the contact sensor, point-by-point circumferential PV isolation was performed for both pairs of veins, using 35W of power and contact force between 10-20 g, until electrogram elimination. Esophageal temperature rise occurred in the posterior segments of the left PVs, where shorter (5- to 10- second) applications with lower power (25-30 W) were performed. Adenosine infusions (18 mg) confirmed PV isolation without dormant conduction.
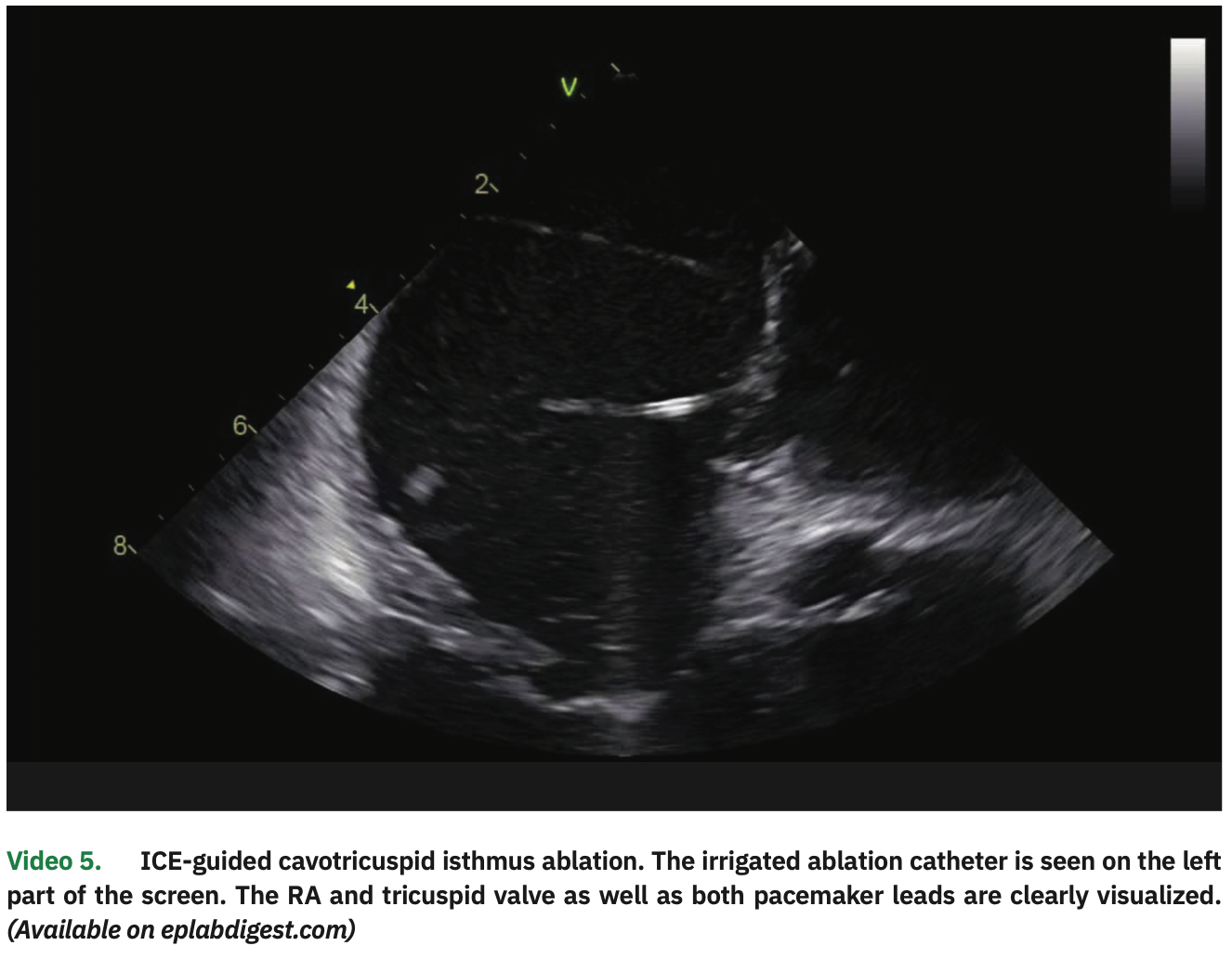
Atrial pacing with 3 extrastimuli (320/320/320 ms) easily induced typical atrial flutter. The ablation catheter was then pulled to the RA, and a linear lesion in the cavotricuspid isthmus (CTI) resulted in interruption (Video 5). Detailed ICE visualization was important to avoid tangling the catheter with the pacemaker leads and to guide tissue contact all along the CTI.
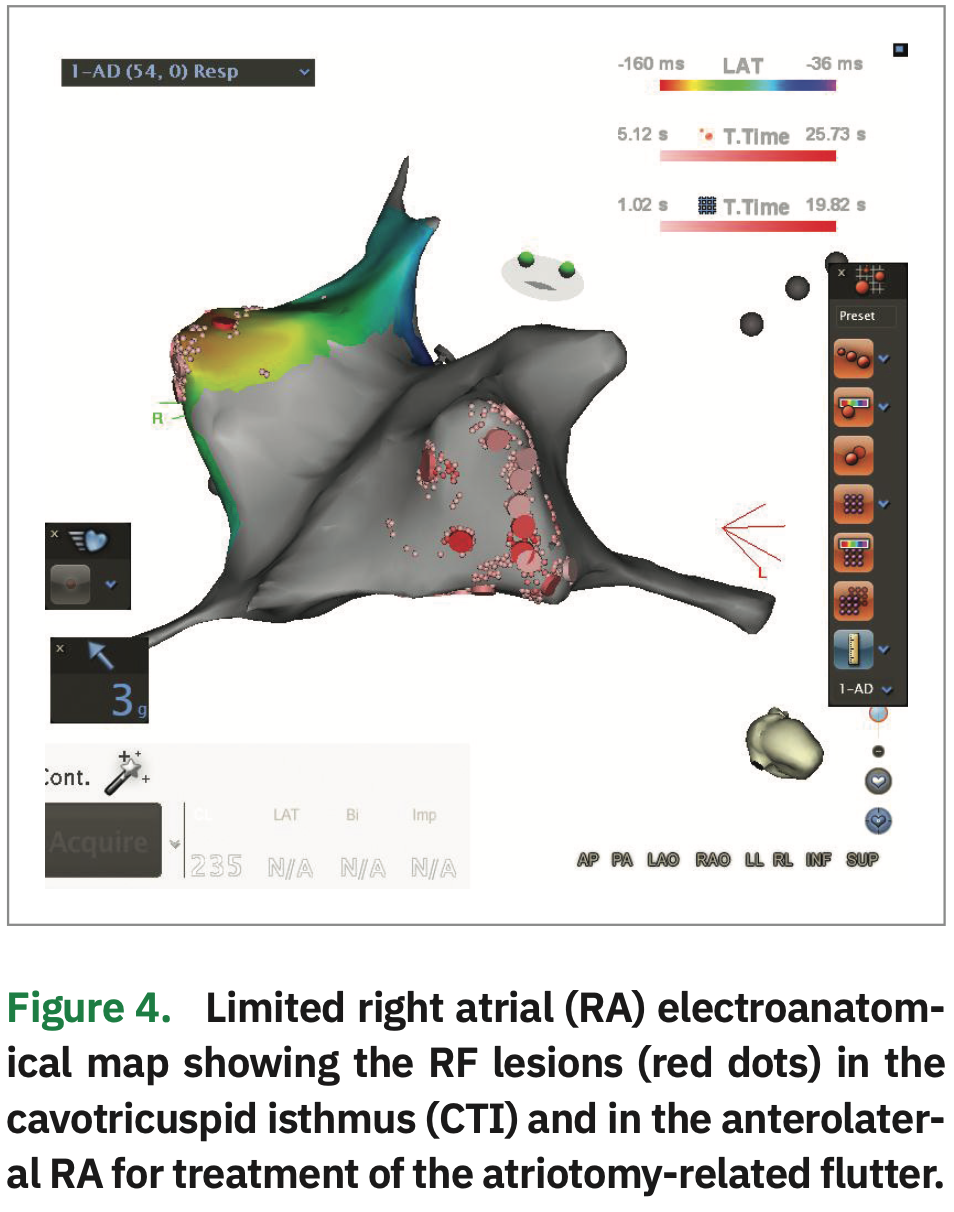
Repeated atrial stimulation resulted in immediate induction of a second morphology of atrial flutter. A left atrial activation map with the multipolar catheter showed bystander activation in the LA. As such, focused RA mapping was performed with the ablation catheter (Figure 4) to find a gap in the previously performed atriotomy (cannulation for extracorporeal circulation). Catheter manipulation was constantly guided by ICE to avoid pulling on the RA lead.
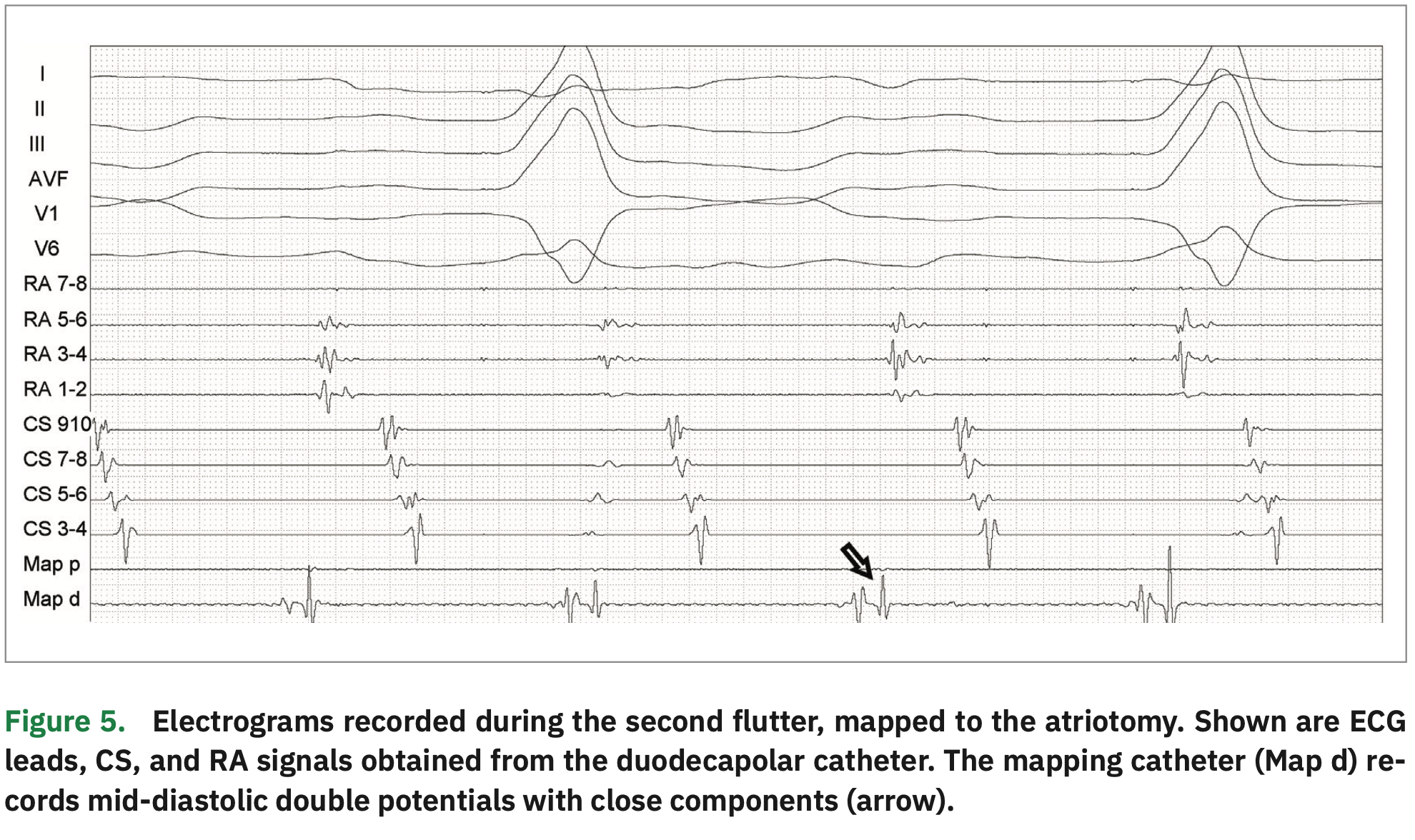
During mapping, the RA atriotomy was defined by the presence of low-voltage areas with separated double potentials in the anterolateral RA. A gap was identified as a mid-diastolic potential with two close components (Figure 5). After high-energy stimulation failed to capture the right phrenic nerve, RF application (30 W) immediately interrupted the arrhythmia. Ablation was extended from the atriotomy to the SVC in order to completely block the circuit.
Repeated atrial stimulation protocol failed to induce any arrhythmias. High-dose isoproterenol infusion (20 mcg/min for 15 minutes) failed to induce non-PV foci. Pacemaker interrogation demonstrated normal lead functioning and thresholds.
Discussion
This case highlights the feasibility, safety, and efficacy of a zero fluoro approach to treat both AF and RA flutters, even in the presence of two pacemaker leads (in a pacer-dependent patient). For that matter, adequate use of ICE and EA mapping are of utmost importance.16-19
In particular, ICE visualization is key in every step of the way. We are even promoting a new hashtag on social media for this (#ICEeyes), since ICE is our “eyes” to see what we are doing. With thorough ICE scanning, all the steps can be adequately monitored, even as catheters come out of the sheath tip (making sure it does not force on the atrial wall). There is no blind step using this approach, even when advancing catheters or wires in the venous system to the heart. CS visualization and cannulation are better than with fluoroscopy. Transseptal punctures are also undoubtedly best visualized on ICE. A short learning curve is required to become comfortable and proficient in ICE manipulation; however, we feel that ICE definitively provides better and more detailed information than fluoroscopy.
EA mapping is standard in most EP labs and familiar to most EP physicians. For zero fluoro procedures, catheter tip color-coding orientation allows for easily reproducible movements as well as sheath positioning. Multipolar catheters allow for less traumatic and higher density maps with better anatomy delineation.
Therefore, all the tools needed for a successful radiation-free ablation are already available. Engagement in this field only needs a motivated team with a change in mindset. Once that occurs, there is no turning back. It is highly beneficial to patients who frequently undergo more than one ablation and who usually have other diagnostic or therapeutic modalities that use radiation (eg, CT scans, coronary interventions) over their lifespan that are usually unaccounted or neglected. We have to keep in mind that the risk is cumulative over time (deterministic effects), especially when cancer statistics are on a worrisome steep rise and when the impact can occur years after exposure, not to mention the worrisome stochastic effects of radiation — effects that occur by chance and which may occur without a threshold level of dose.
Radiation-free interventions also allow for safe ablation of pregnant patients. In fact, the most recently published ESC guidelines for treatment of supraventricular arrhythmias20 gives a IIa indication in experienced centers.
Finally, zero fluoro is also highly beneficial to the health care team. Reducing radiation exposure is desirable for people who face long-term daily exposure. Reports show an increase of up to 1% in the lifetime risk of cancer;3,7 importantly, 85% of brain cancers in interventional physicians occur on the left hemisphere,21-23 suggesting a causal relation of occupational exposure to radiation effects (since the left side is known to be more exposed than the right). There is also the benefit of not using heavy lead aprons, which can make orthopedic issues an almost unanimous occurrence.24-26
Summary
Zero fluoro AF ablation is a win-win situation. It is highly beneficial to patients and the health care team. It can be performed with similar safety and effectiveness as standard fluoro-based procedures, even when additional hardware is implanted in the heart. When ICE and EA mapping systems are used accurately and efficiently, all the steps can be clearly visualized. Catheter positioning and manipulation can be guided with more detail than two-dimensional fluoroscopy can provide. A change in mindset needs to take place. Once you do it, there is no way back!
Disclosures: Dr. Saad has no conflicts of interest to report regarding the content herein.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151.
- Heidbuchel H, Wittkampf FH, Vano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. 2014;16(7):946-964.
- Perisinakis K, Damilakis J, Theocharopoulos N, et al. Accurate assessment of patient effective radiation dose and associated detriment risk from radiofrequency catheter ablation procedures. Circulation. 2001;104(1):58-62.
- Lickfett L, Mahesh M, Vasamreddy C, et al. Radiation exposure during catheter ablation of atrial fibrillation. Circulation. 2004;110(19):3003-3010.
- Ector J, Dragusin O, Adriaenssessns B, et al. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol. 2007;50(3):234-242.
- Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849-857.
- Bourier F, Reents T, Ammar-Busch S, et al. Evaluation of a new very low dose imaging protocol: feasibility and impact on x-ray dose levels in electrophysiology procedures. Europace. 2016;18(9):1406-1410.
- Durán A, Hian SK, Miller DL, Le Heron J, Padovani R, Vano E. Recommendations for occupational radiation protection in interventional cardiology. Catheter Cardiovasc Interv. 2013;82(1):29-42.
- Lerman BB, Markowitz SM, Liu CF, Thomas G, Ip JE, Cheung JW. Fluoroless catheter ablation of atrial fibrillation. Heart Rhythm. 2017;14(6):928-934.
- Ferguson JD, Helms A, Mangrum JM, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2(6):611-619.
- Reddy VY, Morales G, Ahmed H, et al. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm. 2010;7(11):1644-1653.
- Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38(7):797-806.
- Razminia M, Willoughby MC, Demo H, et al. Fluoroless catheter ablation of cardiac arrhythmias: a 5-year experience. Pacing Clin Electrophysiol. 2017;40(4):425-433.
- Yang L, Sun G, Chen X, et al. Meta-analysis of zero or near-zero fluoroscopy use during ablation of cardiac arrhythmias. Am J Cardiol. 2016;118(10):1511-1518.
- Enriquez A, Saenz LC, Rosso R, et al. Use of intracardiac echocardiography in interventional cardiology: working with the anatomy rather than fighting it. Circulation. 2018;137(21):2278-2294.
- Saad EB, Costa IP, Camanho LE. Use of intracardiac echocardiography in the electrophysiology laboratory. Arq Bras Cardiol. 2011;96(1):e11-17.
- Rolf S, Hindricks G, Sommer P, et al. Electroanatomical mapping of atrial fibrillation: review of the current techniques and advances. J Atr Fibrillation. 2014;7(4):1140.
- Nedios S, Sommer P, Bollmann A, Hindricks G. Advanced mapping systems to guide atrial fibrillation ablation: electrical information that matters. J Atr Fibrillation. 2016;8(6):1337.
- Brugada J, Katritsis DG, Arbelo E, et al. 2019 guidelines for the management of patients with supraventricular tachycardia. Eur Heart J. 2019; 00:1-65.
- Blettner M, Schlehofer B, Samkange-Zeeb F, Berg G, Schlaefer K, Schuz J. Medical exposure to ionising radiation and the risk of brain tumours: Interphone study group, Germany. Eur J Cancer. 2007;43(13):1990-1998.
- Carozza SE, Wrensch M, Miike R, et al. Occupation and adult gliomas. Am J Epidemiol. 2000;152(9):838-846.
- Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111(9):1368-1372.
- Klein LW, Miller DL, Balter S, et al. Occupational health hazards in the interventional laboratory: time for a safer environment. Radiology. 2009;250(2):538-544.
- Goldstein JA, Balter S, Cowley M, et al. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63(4):407-411.
- Ross AM, Segal J, Borenstein D, Jenkins E, Cho S. Prevalence of spinal disc disease among interventional cardiologists. Am J Cardiol. 1997;79(1):68-70.