A U.S. Multicenter Examination of the New Cryoballoon: Early Experience With Procedural Enhancements for the Treatment of Atrial Fibrillation
Introduction
Recently, vast improvements have been made in the diagnosis, care, and treatment of atrial fibrillation (AF). Within the pharmaceutical therapies for AF, improved drugs have been created that better control rhythm, rate, and anticoagulation.1 However, there are still a substantial amount of highly symptomatic, drug refractory AF patients; in these patients, cardiac ablation of the focal asynchronous AF electrical source is often the only treatment available to alleviate or terminate the arrhythmia and its associated symptoms. When AF ablation is warranted, pulmonary vein isolation (PVI) is a central pillar to most AF ablation procedures.2 As much as 94% of the foci that are attributed to AF appear to originate from the pulmonary veins (PVs).3
When AF is manifested through PV triggers rather than differential electrical conductance in the atrial substrate or via macroreentry pathways, single shot ablation products (cryoballoon, laser balloon, mesh ablator, and circular phased RF) can facilitate isolation of focal PV triggers. In the U.S., the only FDA-approved single shot ablation product is the cryoballoon, which has undergone a second-generation improvement (Arctic Front Advance™, Medtronic, Inc.). The main improvement in the second-generation cryoballoon is the addition of four internal refrigerant ports (eight total ports) that disperse the liquid nitrous oxide within the cryoballoon across a wider surface area which maintains a more uniform cryoballoon surface temperature (Figure 1). A more detailed description of the cryoballoon improvements has been previously reported.4
The new catheter design intent was to minimize the potential repositioning that may be necessary to achieve an effective cryoballoon isolation of a PV. As a direct consequence, it is potentially possible that further efficiencies would be created in time savings, usage of radiation, and use of cryoballoon catheters. Our intent was to further examine the new cryoballoon beyond the first German evaluation which was limited to 60 patients.4 In this study, we employed a registry-style evaluation to compare the two cryoballoon catheters with regard to procedure time, left atrial (LA) dwell time, fluoroscopy time, mean number of cryoballoons used per procedure, and mean number of cryoablation applications per vein.
Design changes in the second-generation cryoballoon permitted a comparison of the two products to determine if the procedure efficiencies were realized and measurable. The purpose of this article is to discuss early procedural data from a multicenter study examining the enhancements of the first- and second-generation cryoballoon for the treatment of AF. As of yet, there are few reported U.S. multicenter evaluations of the cryoballoon beyond the original STOP AF (Sustained Treatment of Paroxysmal Atrial Fibrillation) clinical trial.5
Methods
 In this procedure monitoring study, eight previous cryoballoon users were asked to share their procedural experience with regard to the new and old cryoballoon (Table 1). These physicians and their respective hospitals represented a geographical mix of regional specialized cardiac hospitals. Throughout this study, no effort was made to harmonize work stream between centers, modify physician approach, or alter patient selection. During the registry collection no instructions were given about patient selection, physician ablation approach, or ablation strategy. In regard to ablation methodology, the physicians represented a wide range of procedural approaches that encompasses the typical usage of the cryoballoon system, including: 1) usage of single or double transseptal puncture; 2) usage or non-usage of the Achieve™ Mapping Catheter (Medtronic, Inc.); 3) usage or non-usage of electroanatomical mapping systems; 4) usage of 23 mm or 28 mm cryoballoon; 5) usage or non-usage of pre-procedural imaging (CT, MRI, or TEE); and 6) usage or non-usage of ICE ultrasound live imaging or pressure monitoring during the procedure.
In this procedure monitoring study, eight previous cryoballoon users were asked to share their procedural experience with regard to the new and old cryoballoon (Table 1). These physicians and their respective hospitals represented a geographical mix of regional specialized cardiac hospitals. Throughout this study, no effort was made to harmonize work stream between centers, modify physician approach, or alter patient selection. During the registry collection no instructions were given about patient selection, physician ablation approach, or ablation strategy. In regard to ablation methodology, the physicians represented a wide range of procedural approaches that encompasses the typical usage of the cryoballoon system, including: 1) usage of single or double transseptal puncture; 2) usage or non-usage of the Achieve™ Mapping Catheter (Medtronic, Inc.); 3) usage or non-usage of electroanatomical mapping systems; 4) usage of 23 mm or 28 mm cryoballoon; 5) usage or non-usage of pre-procedural imaging (CT, MRI, or TEE); and 6) usage or non-usage of ICE ultrasound live imaging or pressure monitoring during the procedure.
Data were collected during the normal work flow of the electrophysiology laboratory by Medtronic clinical support staff on standardized forms submitted electronically to a central Medtronic database. Study recordings were taken anytime a Medtronic staff employee was present during the ablation procedure. However, the first ten cases of Arctic Front usage (by any physician) were not recorded in this study in order to eliminate or minimize the extra variability in time measurements to the Arctic Front data set that may be attributed to an initial product learning curve (specifically, the amount of practice needed to be proficient at over-the-wire balloon deployment). Conversely, in the Arctic Front Advance experience, the first ten cases were included, as physicians by this time period in our study required no additional training to be able to expertly handle the new cryoballoon. In particular, the over-the-wire delivery technique remains unchanged between both generations of the cryoballoon. Without the removal of the data during the learning curve (the first ten cases with the first-generation cryoballoon), some of the differences between the first- and second-generation cryoballoon may have simply been attributed to “learning” how to handle a single shot ablation catheter.
During this study collection, uniform terminology was employed between centers. Procedure time was measured as “skin-to-skin” time, which encompassed from the first catheter entrance into the patient until the last catheter exit. The LA dwell time was denoted as “septum-to-septum” time, which was the total time between the first transseptal entrance to the last transseptal exit time. Fluoroscopy time was measured in minutes and recorded off the EP lab notes. The mean number of cryoballoons per procedure and the mean number of cryoablation applications per vein were recorded by Medtronic staff during the procedures. Throughout all procedural annotations, only anonymised data were used for the analyses.
Descriptive statistics were employed, with all data presented as means with reported standard errors. Statistical comparisons were tested by two-sample student’s t-test with statistical significance established at p-values <0.05.
Results
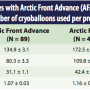 The results of this study are reported in Tables 2 and 3. There were more total Arctic Front procedures that were recorded compared to Arctic Front Advance cases; simply, the Arctic Front Advance catheter has not yet been available in the market for an equivalent time compared to the original Arctic Front system during the course of this study collection. There were 89 case recordings for the Arctic Front Advance and 410 procedural recordings for the Arctic Front in Table 2. As demonstrated in Table 2, there was a statistically significant 21.8% decrease in procedure time from Arctic Front to Arctic Front Advance. There was also a statistically significant
The results of this study are reported in Tables 2 and 3. There were more total Arctic Front procedures that were recorded compared to Arctic Front Advance cases; simply, the Arctic Front Advance catheter has not yet been available in the market for an equivalent time compared to the original Arctic Front system during the course of this study collection. There were 89 case recordings for the Arctic Front Advance and 410 procedural recordings for the Arctic Front in Table 2. As demonstrated in Table 2, there was a statistically significant 21.8% decrease in procedure time from Arctic Front to Arctic Front Advance. There was also a statistically significant  26.9% decrease in LA dwell time and a 26.7% decrease in fluoroscopy time when moving to the new Arctic Front Advance catheter. Additionally, the mean number of cryoballoons used per procedure decreased by 7.8% from 1.16 to 1.07 when the new cryoballoon was employed. Of significance in Table 2, an average of approximately 38 minutes was effectively removed from the procedure time when using the new cryoballoon and an average of about 30 minutes was reduced in LA dwell time.
26.9% decrease in LA dwell time and a 26.7% decrease in fluoroscopy time when moving to the new Arctic Front Advance catheter. Additionally, the mean number of cryoballoons used per procedure decreased by 7.8% from 1.16 to 1.07 when the new cryoballoon was employed. Of significance in Table 2, an average of approximately 38 minutes was effectively removed from the procedure time when using the new cryoballoon and an average of about 30 minutes was reduced in LA dwell time.
Table 3 shows the mean number of cryoablation applications per vein. Of note, there was a significant decrease in the mean number of cryoablation applications on the left-sided veins when moving to the new Arctic Front Advance catheter; however, the right-sided veins were not statistically different. There was a greater than 16% reduction in the mean number of cryoablation applications on the left-sided veins when using the new cryoballoon catheter. Comparisons were not made on the left common ostium and right middle veins because the infrequent occurrences did not allow for statistically meaningful analyses.
When examining the left-sided PVs, there was about a one-unit total reduction in cryoablation applications when moving to the Arctic Front Advance (LSPV=0.50 and LIPV=0.52). In a typical procedure, each additional cryoablation application will last from approximately four to six minutes. Consequently, the 30-minute savings in LA dwell time suggests that the time savings were attributed to more than the reduction in cryoablation applications. It suggests that physicians might have also spent less time manipulating the cryoballoon into position and/or perhaps spent less time during the freeze application within each freeze.
Lastly, during the study data recordings, the Medtronic clinical staff reported the number of times that a RF ablation catheter was additionally used with the cryoballoon to successfully create a PVI. During the usage of the Arctic Front catheter, 16 of the 410 patients required additional usage of a RF ablation catheter to achieve complete PVI (3.9%). By comparison, 2 of the 89 (2.2%) patients treated with the Arctic Front Advance cryoballoon required the additional usage of a RF catheter to achieve total PVI.
Discussion
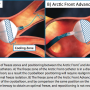 In this registry examination, real and measurable benefits in procedural efficiencies were detected when comparing the new cryoballoon to the original Arctic Front catheter. The second-generation cryoballoon enabled significant time savings in procedure time, LA dwell time, and a reduced duration of fluoroscopy exposure. These benefits were likely due to the simple, fast, and effective cryoballoon placement and freeze application that was achieved by the new Arctic Front Advance cardiac catheter system. Primarily, the new cryoballoon does not require the near perfect axial alignment to the PVs that was needed in the original cryoballoon to achieve effective cryolesion formation (Figure 1).
In this registry examination, real and measurable benefits in procedural efficiencies were detected when comparing the new cryoballoon to the original Arctic Front catheter. The second-generation cryoballoon enabled significant time savings in procedure time, LA dwell time, and a reduced duration of fluoroscopy exposure. These benefits were likely due to the simple, fast, and effective cryoballoon placement and freeze application that was achieved by the new Arctic Front Advance cardiac catheter system. Primarily, the new cryoballoon does not require the near perfect axial alignment to the PVs that was needed in the original cryoballoon to achieve effective cryolesion formation (Figure 1).
This study is the first U.S. multicenter experience reporting of the new Arctic Front Advance system, and our findings are similar to those reported in a recent publication by two German centers using similar metrics.4 In the German study, procedure time decreased by 23% with the introduction of the Arctic Front Advance, in comparison to 21.8% in this study. Similarly, the German study found a 31% decrease in fluoroscopy time when using the new cryoballoon, compared to our 26.7% decrease. Also in our assessment, the number of cryoablation applications per vein decreased from an average of 2.67 to 2.35 applications per PV. Despite variations in technique and ablation strategies in the two studies, overall results were remarkably similar.
As a likely consequence of the “more forgiving” cryoballoon placement and freeze application, there was a significant reduction in fluoroscopy time used per procedural case. Fluoroscopy usage was reduced by more than ten minutes. In our experience with the new system (unlike the first-generation cryoballoon), axial alignment with the PV is not as critical. This probably resulted in less PV rewiring and fewer repeated attempts at occlusion, both of which could have led to reduced fluoroscopy. Such reductions in radiation exposure benefit both patient and staff and are in line with other modifications to the modern laboratory environment intent on reducing the radiation hazard.6
In regard to the number of cryoablation applications per vein, it was particularly interesting to find the differences between left-sided and right-sided veins. There was a reduction in the mean number of cryoablation applications per vein that was apparent on the left-sided veins but not differentiated on the right-sided veins when comparing Arctic Front to Arctic Front Advance. It is important to also note that the left-sided PVs generally had more cryoablation applications than the right-sided veins. The likely reason for this differential cryoapplication is probably a multifactorial combination of many factors, including phrenic nerve palsy (PNP), differential wait time, left PV cross-talk, and differential PV triggers.
PNP is a well-described potential complication of cryothermal ablation, and the systematic review of the cryoablation literature recorded the frequency of transient PNP as being observed in 6.38% of cases.7 Physicians are instructed to monitor PNP by pacing the diaphragm during right-sided ablations, and there is a conservative approach to ablating the right-sided veins with an attempt to keep total ablations close to two (a freeze-thaw-freeze). Consequently, the new cryoballoon has little room for improvement beyond the approximate 2.4 cryoablations per right-sided vein that was established with the original cryoballoon.
The other three factors (differential wait time, left PV cross-talk, and differential PV triggers) are all elements that could lead to a greater number of cryoapplications on the left-sided veins. Since physicians typically begin cryoballoon ablation procedures by ablating the left-sided veins before the right-sided veins, the left-sided veins have more time to recover from conduction dormancy if PV isolation was incomplete, thus necessitating further ablation. Also, cross-talk between the border of the LSPV and LIPV was an observed complexity to ablation in the first-generation cryoballoon that required extra manipulation techniques and cryoablations.8 Lastly, left-sided veins have been observed to have more focal AF triggers than PVs on the right side, as reported in the original work done by the Haïssaguerre group in which the predominance of AF by ectopic beats originated in the left PVs.3 Consequently, it is not too surprising that the Arctic Front Advance cryoballoon catheter demonstrated the most advantage on the most problematic veins.
Gains in safety, efficacy, and efficiency will continue as the entire electrophysiology field gains greater clinical understanding of AF. Studies in recent years have already demonstrated where the focal AF triggers reside,3 what ablation strategies are effective,2,9 and even which repeat ablation procedures are most successful.10 With regard to the cryoballoon, there is additional ongoing research in hospital resource utilization during AF ablation.11 During this current registry examination of the new cryoballoon, an increase in procedural efficiency was observed. Other new product introductions, including a new sheath that is more deflectable (FlexCath Advance™; Medtronic, Inc.), also warrant future investigation.
Limitation
This is a registry examination in which there were no recordings or standardization of several variables, including: patient’s AF disease state, ablation strategy employed, or patient’s previous AF treatment.
Additional information
Data use agreements for this article were provided. No protected health information (PHI) was collected. In this database we do not have PHI by HIPAA guidelines. We did not collect: 1) patient name, 2) Email address, 3) Address, 4) Relatives’ names, 5) Birth date, 6) IP address, 7) Medical record number, 8) Ages, 9) Social security number, 10) Device serial number, 11) Health plan beneficiary, 12) Employer, 13) Banking information, 14) Licence number, 15) Photographs, 16) Biometric identifiers, 17) Telephone or Fax number, or 18) Any unique identifiers.
Disclosures
Neither physicians nor hospitals were paid for their participation in this registry study. Hae Lim, PhD declares a competing financial interest; he is an employee of Medtronic, Inc., a publicly traded company. Eric Johnson, MD reports consultancy and payment for development of educational presentations including service on speakers’ bureaus (education and training). Hanscy Seide, MD has no disclosures to report. F. Kevin Hackett, MD reports board membership with Medtronic on an independent physician quality panel, and reports that travel/accommodation expenses have been covered or reimbursed for this panel. J. Thomas Svinarich, MD reports receiving honoraria and payment for development of educational presentations including service on speakers’ bureaus from Medtronic in regards to the Arctic Front Masters Program (AFMP) faculty; he also reports honoraria from Forest Pharmaceuticals and Sanofi-Aventis. Stephen Stark, MD has no disclosures to report. James Irwin, MD reports receiving payment for development of educational presentations including service on speakers’ bureaus (regarding training staff for cryoballoon). J. Brian DeVille, MD reports consultancy (instruction, cryoablation; research support, other study; instruction, pacemaker), honoraria, payment for development of educational presentations including service on speakers’ bureaus, and travel/accommodation expenses have been covered or reimbursed from Medtronic. Dan Dan, MD reports consultancy, honoraria, payment for development of educational presentations including service on speakers’ bureaus, and travel/accommodation expenses have been covered or reimbursed from Medtronic; he also reports grants/grants pending to his institution through Sorin, and that travel/accommodation expenses have been covered or reimbursed from St. Jude Medical.
Editor's note: This article underwent peer review by one or more members of EP Lab Digest's editorial board.
References
- Prystowsky EN, Benson DW Jr, Fuster V, et al. Management of patients with atrial fibrillation. A Statement for Healthcare Professionals. From the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996;93:1262-1277.
- Calkins H, Kuck KH, Cappato R, et al; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632-696.
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666.
- Fürnkranz A, Bordignon S, Schmidt B, et al. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol. 2012 Dec 24. [Epub ahead of print].
- Packer DL, Kowal RC, Wheelan KR, et al; STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013 Mar 21. [Epub ahead of print].
- Pantos I, Koukorava C, Nirgianaki E, et al. Radiation exposure of the operator during cardiac catheter ablation procedures. Radiat Prot Dosimetry. 2012;150:306-311.
- Andrade JG, Khairy P, Guerra PG, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8:1444-1451.
- Chun KR, Schmidt B, Metzner A, et al. The ‘single big cryoballoon’ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur Heart J. 2009;30:699-709.
- Raviele A, Natale A, Calkins H, et al; for the Venice Chart members. Venice Chart International Consensus document on atrial fibrillation ablation: 2011 Update. J Cardiovasc Electrophysiol. 2012;23:890-923.
- Schade A, Langbein A, Spehl S, et al. Recurrence of paroxysmal atrial fibrillation after cryoisolation of the pulmonary veins. Is a “redo” procedure using the cryoballoon useful? J Interv Card Electrophysiol. 2012 Nov 7. [Epub ahead of print].
- DeVille JB, Svinarich JT, Dan D, et al. Comparison of resource utilization for PVI: cryoablation versus RF with three-dimensional mapping. 2013 Boston AF conference.











