Unusual Case of Atrial Fibrillation: Noncompaction of the Left Ventricle
Case Presentation
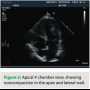
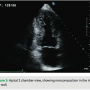
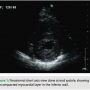 A 39-year-old female presented to the emergency department with chest pain and palpitations. She was hiking with her husband when the palpitations started and she felt her pulse, which seemed to be fast and irregular. Her pain was in the center chest, sharp with no radiation, but she felt fatigued and tired. She reports a history of being short of breath in the past two months during exercise. Her father died suddenly at age 30 years, but never had an autopsy done. She takes no medications and works as a nurse. Her physical examination revealed a heart rate of 160 beats per minute and her blood pressure was 110/65. Her pulse was irregular, and her ECG revealed atrial fibrillation with left ventricular (LV) hypertrophy. Her labs were normal, and her troponin level was 0.29 ng/mL. An echocardiogram was done and revealed increased LV wall thickness, and she was diagnosed with hypertrophic cardiomyopathy. Her ejection fraction was noted to be 40% with wall motion abnormalities. She was transferred to our center, and by the time she arrived, she was in sinus rhythm. She underwent left heart catheterization, which showed normal coronary arteries. When reviewing her echocardiogram, heavy trabeculations were noted in the apex, inferior wall, as well as the lateral wall (Figures 1–3). She was diagnosed with noncompaction of the left ventricle, with a ratio of noncompacted to compacted myocardium of 2.5/1. She had no other congenital heart defects except for patent foramen ovale.
A 39-year-old female presented to the emergency department with chest pain and palpitations. She was hiking with her husband when the palpitations started and she felt her pulse, which seemed to be fast and irregular. Her pain was in the center chest, sharp with no radiation, but she felt fatigued and tired. She reports a history of being short of breath in the past two months during exercise. Her father died suddenly at age 30 years, but never had an autopsy done. She takes no medications and works as a nurse. Her physical examination revealed a heart rate of 160 beats per minute and her blood pressure was 110/65. Her pulse was irregular, and her ECG revealed atrial fibrillation with left ventricular (LV) hypertrophy. Her labs were normal, and her troponin level was 0.29 ng/mL. An echocardiogram was done and revealed increased LV wall thickness, and she was diagnosed with hypertrophic cardiomyopathy. Her ejection fraction was noted to be 40% with wall motion abnormalities. She was transferred to our center, and by the time she arrived, she was in sinus rhythm. She underwent left heart catheterization, which showed normal coronary arteries. When reviewing her echocardiogram, heavy trabeculations were noted in the apex, inferior wall, as well as the lateral wall (Figures 1–3). She was diagnosed with noncompaction of the left ventricle, with a ratio of noncompacted to compacted myocardium of 2.5/1. She had no other congenital heart defects except for patent foramen ovale.
Discussion
 Noncompaction of the left ventricular myocardium is a rare disorder that happens in isolation or with other congenital cardiac defects. It is caused by the arrest of compaction of the myocardial fibers, leading to prominent trabeculations and spongy appearance of the myocardium. Often it is associated with other congenital cardiac anomalies, especially obstruction of the right or left ventricular outflow tracts, the deep intertrabecular recesses that persist in these cases are in communication with the ventricular cavity and the coronary circulation. In contrast, the intertrabecular recesses in isolated LV noncompaction are in communication with the LV cavity only and not with the coronary circulation. Biopsies of patients with isolated LV noncompaction (ILVNC) show interstitial and subendocardial fibrosis as well as endomyocardial thickening.1 There is microcirculatory dysfunction in both compacted and noncompacted segments. This might explain the wall motion abnormalities noted in imaging studies and subendocardial scars noted in histological preparations.2
Noncompaction of the left ventricular myocardium is a rare disorder that happens in isolation or with other congenital cardiac defects. It is caused by the arrest of compaction of the myocardial fibers, leading to prominent trabeculations and spongy appearance of the myocardium. Often it is associated with other congenital cardiac anomalies, especially obstruction of the right or left ventricular outflow tracts, the deep intertrabecular recesses that persist in these cases are in communication with the ventricular cavity and the coronary circulation. In contrast, the intertrabecular recesses in isolated LV noncompaction are in communication with the LV cavity only and not with the coronary circulation. Biopsies of patients with isolated LV noncompaction (ILVNC) show interstitial and subendocardial fibrosis as well as endomyocardial thickening.1 There is microcirculatory dysfunction in both compacted and noncompacted segments. This might explain the wall motion abnormalities noted in imaging studies and subendocardial scars noted in histological preparations.2
 Isolated LV noncompaction was first described in 1990 by Chin et al, with 50% familial involvement.3 In later series involving adults, the familial recurrence ranged from 12–44%. Ichida et al reported novel mutations in the G4.5 gene and mutations in the α-dystrobrevin gene, which is associated with muscular dystrophy in humans. Blyle et al found a mutation of the G4.5 gene of the Xq28 chromosome region to be responsible of isolated LV noncompaction in a family with six affected children. This chromosome region is also responsible for other myopathies including Barth Syndrome.4
Isolated LV noncompaction was first described in 1990 by Chin et al, with 50% familial involvement.3 In later series involving adults, the familial recurrence ranged from 12–44%. Ichida et al reported novel mutations in the G4.5 gene and mutations in the α-dystrobrevin gene, which is associated with muscular dystrophy in humans. Blyle et al found a mutation of the G4.5 gene of the Xq28 chromosome region to be responsible of isolated LV noncompaction in a family with six affected children. This chromosome region is also responsible for other myopathies including Barth Syndrome.4
The true prevalence of isolated LV noncompaction is unknown, as most cases are referred to tertiary care centers. In reported series, males are more commonly affected than females. Patients with isolated LV noncompaction can be asymptomatic for years and eventually could present with heart failure, arrhythmias and/or embolic events. Most patients will have some degree of LV dysfunction, which has been reported in more than 60% of patients in the four largest reports of LV noncompaction.3,5-7 However, presentation as congestive heart failure with dyspnea on exertion has ranged from 30–68%. Both systolic and diastolic dysfunctions have been described, and could be due to abnormal relaxation and restrictive filling from the prominent trabeculations. Moreover, the microcirculatory dysfunction found in both compacted and noncompacted segments can lead to wall motion abnormalities, impaired contractile function and abnormalities in diastolic function. Several arrhythmias have been reported with ILVNC, including atrial fibrillation (5–29% in major reports), atrial tachycardia, premature ventricular complexes and ventricular tachycardia (18–47% in major reports). Sudden cardiac death accounted for 50% of deaths in ILVNC. Presence of subendocardial scar can act as a substrate of reentry in these patients. Triggered mechanism has also been suggested.8 Other abnormalities noted in the electrocardiogram include left bundle branch block, right bundle branch block, left ventricular hypertrophy with repolarization abnormalities, and AV block. In children, Wolff-Parkinson-White syndrome has been described as well. Embolic complications in ILVNC could be due to thrombus formation in the recesses of the trabeculations, due to stagnant flow from severely depressed LV function or from the presence of atrial fibrillation. These emboli can go to the cerebrovascular circulation, peripheral circulation, or pulmonary circulation. The incidence of embolization has ranged from 21–38%. Anticoagulation to prevent thromboembolic complications is very important in ILVNC.1
Two-dimensional echocardiography is the modality most commonly used to diagnose ILVNC. Criteria for diagnoses have been proposed by Chin et al, with a ratio of noncompacted to compacted LV myocardium of 2 to 1 considered diagnostic. This is typically measured at end systole in the parasternal short axis view. The noncompacted segments involve usually the apex more than the base, and are seen mostly in the inferior wall and also in the lateral wall. The right ventricle is involved in 40% of the cases. Wall motion abnormalities, impaired diastolic filling as measured from mitral inflow velocities are also seen. Depressed LV ejection fraction is noted in a lot of patients with LV noncompaction. It is important to differentiate ILVNC from hypertrophic cardiomyopathy (especially the apical variant), dilated cardiomyopathy, arrhythmogenic right ventricular dysplasia and endocardial fibroelastosis. Magnetic resonance imaging (MRI) has been also used for diagnosis. Delayed gadolinium enhancement has been seen in both compacted and noncompacted myocardium. In compacted myocardium, delayed enhancement correlated well with fibrosis, while in the noncompacted segments, delayed enhancement correlated with fibrosis as well as mucoid degeneration of the endocardium.9 MRI offers better spatial resolution and can help assess LV and RV functions, wall motion abnormalities, as well as the ratio of compacted and noncompacted segments, which has been shown to be an important predictor of major adverse cardiac events.
Management of patients with ILVNC involves treating heart failure, protection from sudden cardiac death, anticoagulation to prevent thromboembolic events, and screening of family members. Beta blockers and angiotensin enzyme inhibitors are used and have been reported to improve symptoms and the LVEF.10 Anticoagulation with coumadin is recommended in all patients, even if they don’t have atrial fibrillation. Electrophysiology testing to predict the risk of sudden cardiac death has not yielded great results. Currently, the decision to implant a defibrillator (ICD) or biventricular defibrillator (BiV ICD) is clear in patients who have survived a cardiac arrest or in patients with LVEF <35% who qualify for an ICD or BiV ICD according to the current guidelines. In a series of 30 patients with ILVNC who received ICDs or BiV ICDs according to the current guidelines, Kobza et al showed that appropriate ICD therapy (either shocks or antitachycardia pacing) occurred in 37% of cases with a mean follow up of 21 ± 16 months. Inappropriate shocks occurred in 13% of cases. In patients who received ICD therapy for primary prevention, 33% had appropriate ICD therapy with mean follow up of 27 ± 33 months. There were no predictors of appropriate ICD therapy. Patients with ILVNC should have family screening to detect other family members who might have the disease.11
The prognosis of ILVNC varies, but in general the disease is progressive, with heart failure leading to death or transplantation occurring in 47% of adults with ILVNC followed for 44 ± 39 months. Oechslin et al reported that certain clinical characteristics are important in non-survivors compared to survivors, including higher LV end-diastolic diameter, NYHA class III–IV heart failure, left bundle branch block, and persistent atrial fibrillation.5 Patients with such clinical characteristics need frequent follow up, with strong consideration for more aggressive treatment.
Conclusions
Our patient was placed on metoprolol succinate and lisinopril. She was started on coumadin, and her echo 12 months after treatment showed improvement in LVEF to 50%. She had an event monitor, which showed premature ventricular contractions but no sustained ventricular tachycardia or atrial fibrillation. Her family was also screened; her brother was diagnosed with ILVNC and was started on beta blockers and ACE inhibitors, but he refused coumadin and is currently on aspirin only. Her son is 13 years old and has “prominent” trabeculations; he was referred to pediatric cardiology. The patient is followed regularly with assessment of her rhythm and cardiac function, and currently has no indication for more aggressive therapy.
References
- Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation 2004:109:2965–2971.
- Jenni R, Wyss CA, Oechslin EN, Kaufmann PA. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol 2002:39:450–454.
- Chin TK, Perloff JK, Williams RG, et al. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990:82:507–513.
- Bleyl SB, Mumford BR, Brown-Harrison MC, et al. Xq28-linked noncompaction of the left ventricular myocardium: Prenatal diagnosis and pathologic analysis of affected individuals. Am J Med Genet 1997:72:257–265.
- Oechslin EN, Attenhofer Jost CH, Rojas JR, et al. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000:36:493–500.
- Ichida F, Hamamichi Y, Miyawaki T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: Long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 1999:34:233–240.
- Ritter M, Oechslin E, Sutsch G, et al. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 1997:72:26–31.
- Derval N, Jais P, O’Neill MD, Haissaguerre M. Apparent idiopathic ventricular tachycardia associated with isolated ventricular noncompaction. Heart Rhythm 2009:6:385–388.
- Chaowu Y, Li L, Shihua Z. Histopathological features of delayed enhancement cardiovascular magnetic resonance in isolated left ventricular noncompaction. J Am Coll Cardiol 2011;58:311–312.
- Toyono M, Kondo C, Nakajima Y, et al. Effects of carvedilol on left ventricular function, mass, and scintigraphic findings in isolated left ventricular non-compaction. Heart 2001:86:E4.
- Kobza R, Steffel J, Erne P, et al. Implantable cardioverter-defibrillator and cardiac resynchronization therapy in patients with left ventricular noncompaction. Heart Rhythm 2010;7:1545–1549.











