Subcutaneous Defibrillator Implantation Tip: Make a Lean Sandwich
The totally subcutaneous implantable defibrillator (S-ICD) has become a proven alternative to the transvenous ICD for patients who do not need bradycardia, antitachycardia, or resynchronous pacing, and has the advantage of not needing hardware in the heart or vasculature. One tradeoff is the relatively large size of the device needed to deliver a sufficiently large shock to defibrillate the heart from outside the thorax. Furthermore, because of continued concerns that the defibrillation energy requirement (DER), commonly referred to as the defibrillation threshold (DFT), in an individual patient can be higher than the energy that the S-ICD can deliver, defibrillation testing at the time of implantation is still considered necessary. Techniques to lower DFTs can be logical but anecdotal.
Much of the early information related to the S-ICD came from early feasibility trials by Dr. Gust Bardy and colleagues, at which time the device was implanted temporarily in patients already undergoing a transvenous device implantation, and various configurations and vectors were tested.1 Based on that data, the system was designed with a generator and single coil, with an implant technique that involves placement of the generator in the midaxillary line with the center of the generator at the level of the sixth rib or at the LV apex based on fluoroscopy or palpation. The lead is tunneled along the left sternum with an anchoring sleeve above the xiphoid.
To better investigate determinants of S-ICD efficacy without the need for human or animal testing, computer models have been developed. In a recently published study, Dr. Kevin Heist partnered with international physician colleagues as well as scientists working for the maker of the S-ICD to develop a computer model to further study the factors that determine the DFT of the device.2 They created a computer model of the thorax using data from the MRI of a single patient with coronary artery disease, who also allowed the investigators to make confirmatory impedance measurements using esophageal electrodes. This allowed them to create a 3-dimensional matrix of small circuit elements with estimated tissue resistances to represent the thoracic anatomy. For example, fat was assigned a resistance of 2,000 Ohms-cm compared to muscle at 225 Ohms-cm. They simulated the delivery of a biphasic shock between the can and the coil based on a fixed 50% tilt, 98-μF capacitor system. The DFTs were estimated to occur at the voltage at which 95% of the ventricular mass has >4 volts/cm of electric field strength.
The authors found that the location of the generator was important, with successful defibrillation being more sensitive to the anteroposterior position than the cranial to caudal location. A more critical and novel finding was that the presence of fat under the can or coil greatly increased the impedance and DFTs. Modeling using a simulated heart that was dilated or hypertrophied did not negate their findings. They concluded that “an S-ICD implantation strategy involving posterior generator location and coil and generator directly over the fascia without underlying fat is likely to markedly lower DFTs with the S-ICD and assist in troubleshooting of patients with unacceptably high DFTs.”2
The computer simulation used to better understand determinants of successful subcutaneous defibrillation has some limitations. These include use of MRI data from only one patient, and its monodomain model. There are more sophisticated bidomain computer models that account for other factors such as myocardial wave propagation and the effect of virtual electrodes. Evidence that this monodomain model is reasonably predictive, however, includes the results of a validation exercise where it was found that the DFTs of a modeled transvenous system were similar to actual clinical experience.
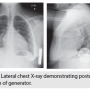 Until more complex computer simulations are employed to study DFTs, the findings from this monodomain model provide guidance on optimal implantation of the current commercially available S-ICD. The figure below shows the lateral chest X-ray of an obese 15-year-old boy with a severe dilated cardiomyopathy who underwent implantation of an S-ICD for primary prevention, demonstrating an optimal posterior position of the generator between the heart and the spine. The modeling data also suggest that when evaluating a patient with an S-ICD who has reached the time for generator replacement for battery depletion, consideration should be made to reposition the new device more posteriorly if it does not appear optimal by exam or radiography. The model could also be used to help develop alternative devices using different lead configurations and implant locations to optimize the shocking vector.
Until more complex computer simulations are employed to study DFTs, the findings from this monodomain model provide guidance on optimal implantation of the current commercially available S-ICD. The figure below shows the lateral chest X-ray of an obese 15-year-old boy with a severe dilated cardiomyopathy who underwent implantation of an S-ICD for primary prevention, demonstrating an optimal posterior position of the generator between the heart and the spine. The modeling data also suggest that when evaluating a patient with an S-ICD who has reached the time for generator replacement for battery depletion, consideration should be made to reposition the new device more posteriorly if it does not appear optimal by exam or radiography. The model could also be used to help develop alternative devices using different lead configurations and implant locations to optimize the shocking vector.
The computer simulation study strongly suggests that for the best results when implanting the current S-ICD device, make a low-fat sandwich. In other words, place the can as posteriorly as possible to sandwich the heart between the lead and generator, and place the device and coil completely under the fat directly on the fascia.
References
- Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36-44.
- Heist EK, Belalcazar A, Stahl W, Brouwer TF, Knops RE. Determinants of Subcutaneous Implantable Cardioverter-Defibrillator Efficacy. A Computer Modeling Study. JACC: Clinical Electrophysiology. 2017;3:405-414.











