Radiofrequency Catheter Ablation of Epicardial Ventricular Tachycardia
In this article, the authors discuss their use of epicardial ablation for ventricular tachycardia at Yale-New Haven Hospital.
 Electroanatomic substrate mapping of the epicardial surface (Carto XP, Biosense Webster, a Johnson & Johnson company, Diamond Bar, CA) demonstrated a large area of very low amplitude electrograms consistent with scar (
Electroanatomic substrate mapping of the epicardial surface (Carto XP, Biosense Webster, a Johnson & Johnson company, Diamond Bar, CA) demonstrated a large area of very low amplitude electrograms consistent with scar ( preceeding the onset of the QRS by approximately 100 ms (Figure 3). Entrainment mapping near that location demonstrated concealed entrainment with a post-pacing interval minus tachycardia cycle length of 15 ms (Figure 1). Coronary angiography showed that the earliest site was several centimeters away from the epicardial coronary arteries (Figure 4). Ablation at this site resulted in immediate VT termination. Radiofrequency energy was delivered at an initial power of 30 W and was gradually titrated
preceeding the onset of the QRS by approximately 100 ms (Figure 3). Entrainment mapping near that location demonstrated concealed entrainment with a post-pacing interval minus tachycardia cycle length of 15 ms (Figure 1). Coronary angiography showed that the earliest site was several centimeters away from the epicardial coronary arteries (Figure 4). Ablation at this site resulted in immediate VT termination. Radiofrequency energy was delivered at an initial power of 30 W and was gradually titrated 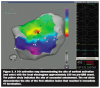 up to 50 W, with a maximal temperature of 42 degrees Celsius and an irrigation flow rate of 17 ml/min. Consolidation lesions were placed in that region. Aspiration of pericardial fluid via the sheath side port was performed after radiofrequency applications in order to avoid accumulation of the irrigant in the pericardial space.
Subsequently, the ablation catheter was withdrawn from the pericardial sheath and
up to 50 W, with a maximal temperature of 42 degrees Celsius and an irrigation flow rate of 17 ml/min. Consolidation lesions were placed in that region. Aspiration of pericardial fluid via the sheath side port was performed after radiofrequency applications in order to avoid accumulation of the irrigant in the pericardial space.
Subsequently, the ablation catheter was withdrawn from the pericardial sheath and  introduced through the femoral artery into the left ventricle in order to map the extent of the endocardial scar. Heparin was administered to maintain an ACT of 300 seconds. The area of endocardial scarring was very small compared to the epicardium, and was limited to a small region that was located transmurally across from the epicardial ablation site (Figure 5). Radiofrequency lesions were placed at that endocardial scar site in order to consolidate the epicardial lesions.
Following ablation, sustained VT could no longer be induced. Heparin was reversed with
introduced through the femoral artery into the left ventricle in order to map the extent of the endocardial scar. Heparin was administered to maintain an ACT of 300 seconds. The area of endocardial scarring was very small compared to the epicardium, and was limited to a small region that was located transmurally across from the epicardial ablation site (Figure 5). Radiofrequency lesions were placed at that endocardial scar site in order to consolidate the epicardial lesions.
Following ablation, sustained VT could no longer be induced. Heparin was reversed with 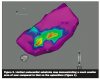 protamine and all sheaths were removed. At the end of the procedure an echocardiogram revealed no residual pericardial fluid. Methylprednisolone (125 mg) was injected into the pericardial space in an effort to reduce post-operative pericarditis.
The patient tolerated the procedure well and returned to his room in stable condition. He did not develop any pericarditis symptoms, and at six-month post-operative follow-up, has not had recurrent VT.
protamine and all sheaths were removed. At the end of the procedure an echocardiogram revealed no residual pericardial fluid. Methylprednisolone (125 mg) was injected into the pericardial space in an effort to reduce post-operative pericarditis.
The patient tolerated the procedure well and returned to his room in stable condition. He did not develop any pericarditis symptoms, and at six-month post-operative follow-up, has not had recurrent VT.
Case History
The patient is a 69-year-old man with a history of non-ischemic cardiomyopathy and multiple hospitalizations for monomorphic ventricular tachycardia (VT). His VT burden became increasingly progressive over the past three years despite treatment with beta blockers and multiple anti-arrhythmic medications including sotalol, dofetilide, and amiodarone. He had recurrent shocks for VT one month prior to presentation. Quinidine was added to amiodarone; however, he subsequently developed syncope associated with multiple ICD shocks, and was referred for catheter ablation. The index of suspicion that this patient’s VT is originating from the epicardial surface was high given the history of dilated non-ischemic cardiomyopathy.Methods
The procedure was performed under general anesthesia. The patient was draped in the usual sterile fashion and sheaths were inserted in the right femoral artery and vein. Access into the pericardial space was achieved using the technique developed by Sosa and colleagues.1 Briefly a subxiphoid percutaneous puncture was performed under fluoroscopic guidance in RAO and LAO views using a 17-gauge epidural needle. Injection of a small amount (~2-3 cc) of contrast demonstrated layering within the pericardial cavity and confirmed access into the pericardial space. A soft-tip hydrophilic guidewire was then advanced and observed to track along the cardiac silhouette crossing multiple chambers, thereby confirming that the puncture was not intracavitary. A 24-cm sheath was subsequently inserted over the wire, and a 3.5-mm externally irrigated tip catheter was advanced into the pericardial space for mapping and ablation. Electroanatomic substrate mapping of the epicardial surface (Carto XP, Biosense Webster, a Johnson & Johnson company, Diamond Bar, CA) demonstrated a large area of very low amplitude electrograms consistent with scar (
Electroanatomic substrate mapping of the epicardial surface (Carto XP, Biosense Webster, a Johnson & Johnson company, Diamond Bar, CA) demonstrated a large area of very low amplitude electrograms consistent with scar ( preceeding the onset of the QRS by approximately 100 ms (Figure 3). Entrainment mapping near that location demonstrated concealed entrainment with a post-pacing interval minus tachycardia cycle length of 15 ms (Figure 1). Coronary angiography showed that the earliest site was several centimeters away from the epicardial coronary arteries (Figure 4). Ablation at this site resulted in immediate VT termination. Radiofrequency energy was delivered at an initial power of 30 W and was gradually titrated
preceeding the onset of the QRS by approximately 100 ms (Figure 3). Entrainment mapping near that location demonstrated concealed entrainment with a post-pacing interval minus tachycardia cycle length of 15 ms (Figure 1). Coronary angiography showed that the earliest site was several centimeters away from the epicardial coronary arteries (Figure 4). Ablation at this site resulted in immediate VT termination. Radiofrequency energy was delivered at an initial power of 30 W and was gradually titrated  up to 50 W, with a maximal temperature of 42 degrees Celsius and an irrigation flow rate of 17 ml/min. Consolidation lesions were placed in that region. Aspiration of pericardial fluid via the sheath side port was performed after radiofrequency applications in order to avoid accumulation of the irrigant in the pericardial space.
Subsequently, the ablation catheter was withdrawn from the pericardial sheath and
up to 50 W, with a maximal temperature of 42 degrees Celsius and an irrigation flow rate of 17 ml/min. Consolidation lesions were placed in that region. Aspiration of pericardial fluid via the sheath side port was performed after radiofrequency applications in order to avoid accumulation of the irrigant in the pericardial space.
Subsequently, the ablation catheter was withdrawn from the pericardial sheath and  introduced through the femoral artery into the left ventricle in order to map the extent of the endocardial scar. Heparin was administered to maintain an ACT of 300 seconds. The area of endocardial scarring was very small compared to the epicardium, and was limited to a small region that was located transmurally across from the epicardial ablation site (Figure 5). Radiofrequency lesions were placed at that endocardial scar site in order to consolidate the epicardial lesions.
Following ablation, sustained VT could no longer be induced. Heparin was reversed with
introduced through the femoral artery into the left ventricle in order to map the extent of the endocardial scar. Heparin was administered to maintain an ACT of 300 seconds. The area of endocardial scarring was very small compared to the epicardium, and was limited to a small region that was located transmurally across from the epicardial ablation site (Figure 5). Radiofrequency lesions were placed at that endocardial scar site in order to consolidate the epicardial lesions.
Following ablation, sustained VT could no longer be induced. Heparin was reversed with  protamine and all sheaths were removed. At the end of the procedure an echocardiogram revealed no residual pericardial fluid. Methylprednisolone (125 mg) was injected into the pericardial space in an effort to reduce post-operative pericarditis.
The patient tolerated the procedure well and returned to his room in stable condition. He did not develop any pericarditis symptoms, and at six-month post-operative follow-up, has not had recurrent VT.
protamine and all sheaths were removed. At the end of the procedure an echocardiogram revealed no residual pericardial fluid. Methylprednisolone (125 mg) was injected into the pericardial space in an effort to reduce post-operative pericarditis.
The patient tolerated the procedure well and returned to his room in stable condition. He did not develop any pericarditis symptoms, and at six-month post-operative follow-up, has not had recurrent VT.
Discussion
Radiofrequency catheter ablation has become an important tool for the successful management of patients with refractory ventricular arrhythmias. Endocardial ablation success rates are variable, ranging from 50-80%,2,3 and nearly one-third of all VT circuits are inaccessible from the endocardium due to epicardial or intramural locations. Thus, epicardial ablation has emerged as an essential modality for VT ablation, especially in patients with non-ischemic cardiomyopathy who are more likely to have epicardial substrates. As mentioned earlier, the index of suspicion that this VT originated from the epicardium was high given the history of dilated non-ischemic cardiomyopathy. Furthermore, his VT demonstrated delayed intrinsicoid deflection consistent with an epicardial origin.7 Accordingly, pericardial access was obtained prior to heparin administration and endocardial mapping. As demonstrated in this case and in contradistinction to patients with ischemic heart disease, the area of epicardial scarring is often much larger than endocardial scar in patients with non-ischemic cardiomyopathy, and it is usually not confined to a specific coronary vascular territory.4-6 Since its introduction by Sosa and colleagues,1 transcutaneous epicardial access has been gaining wider acceptance. Epicardial substrates have been increasingly recognized in all types of VT including infarct/scar related VT, idiopathic VT, arrhythmogenic right ventricular dysplasia, and non-ischemic cardiomyopathy.4-8 A recent study9 reported that approximately 13% of patients with VT require epicardial ablation. The highest prevalence of epicardial VT circuits is in patients with arrhythmogenic right ventricular cardiomyopathy (41%) and nonischemic cardiomyopathy (35%), followed by ischemic heart disease (16%). Thus, it is becoming increasingly apparent that epicardial involvement may be higher than previously appreciated, especially in certain substrates and in the setting of previously failed endocardial ablation. The percutaneous subxiphoid approach to access the pericardium remains challenging. However, in experienced hands, epicardial VT ablation can be performed effectively and safely. Failure to obtain epicardial access occurs in approximately 10% of cases and is most often related to pericardial adhesions in the setting of a history of cardiac surgery. Diagnostic mapping is performed in a similar fashion to endocardial mapping. However, pace- and entrainment mapping may be limited by poor capture thresholds due to the presence of pericardial fat. Similarly, it may be difficult to differentiate low voltage due to true scar from that due to epicardial fat, and this may limit substrate mapping. In general, electrograms in areas of scar demonstrate low voltage (9 reported that 71% of patients were free from VT recurrence after a mean follow-up period of 23 months. This is similar to other published case series reporting success rates from 63% to 78%.4,6,8 Major complications include hemopericardium as well as injury to subdiaphragmatic vessels and damage to the ventricles, epicardial coronary arteries or left phrenic nerve. In the retrospective multicenter safety study by Sacher et al, acute major complications (mainly pericardial bleeding that required intervention) occurred in 5%, and delayed complications occurred in 2%.9 Coronary angiography is usually performed prior to radiofrequency lesion application in order to ascertain the distance from coronary vessels. Although data are lacking, a distance of ≥ 1 cm from the ablation catheter to the coronary artery is generally considered to be relatively safe. In addition, high-output pacing is performed prior to ablation on the lateral left ventricular wall in order to avoid injury to the left phrenic nerve. Pericarditic discomfort is very common following the procedure, leading some investigators to inject glucocorticoids in the pericardial space prior to catheter removal in hopes of preventing pericarditis and reducing subsequent fibrosis. The presence of pericardial adhesions, as is seen following cardiac surgery, can significantly hinder access and catheter movement. These patients often need a direct surgical approach to the pericardial space. In conclusion, catheter epicardial VT ablation is emerging as a practical alternative to conventional endocardial catheter ablation of VT in certain selected patients. With more experience, substrate recognition, and appropriate patient selection, this procedure is likely to gain wider acceptance as a safe and effective treatment option for patients with incessant VT that might not otherwise respond to an endocardial approach alone.References
- Sosa E, Scanavacca M, d’Avila A, Pilleggi E. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol 1996;7:531–536.
- Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: The multicenter thermocool ventricular tachycardia ablation trial. Circulation 2008;118:2773–2782.
- Tanner H, Hindricks G, Volkmer M, et al. Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: Results of the prospective multicenter Euro-VT-study. J Cardiovasc Electrophysiol 2010;21:47–53.
- Soejima K, Stevenson W, Sapp J, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: The importance of low-voltage scars. J Am Coll Cardiol 2004;43:1834–1842.
- Cesario D, Vaseghi M, Boyle N, et al. Value of high-density endocardial and epicardial mapping for catheter ablation of hemodynamically unstable ventricular tachycardia. Heart Rhythm 2006;3:1–10.
- Cano O, Hutchinson M, Lin D, et al. Electro-anatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009;54:799–808.
- Daniels D, Lu Y, Morton J, et al. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: Electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation 2006;113:1659–1666.
- Garcia F, Bazan V, Zado E, et al. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120: 366–375.
- Sacher F, Roberts-Thomson K, Maury P, et al. Epicardial ventricular tachycardia ablation: A multicenter safety study. J Am Coll Cardiol 2010;55:2366–2372.











