ADVERTISEMENT
A Novel, Fully Absorbable Hemostatic Agent (From Purified Plant Starch) With Desiccant Properties for Implantable Device Pocket Management
Device pocket hematomas continue to be one of the banes of the implanter’s existence. They are a complication of device implants, upgrades, and generator changeouts, with an incidence reported at anywhere from 2-20%. In a very thorough review of the management of antithrombotic therapy in patients undergoing device surgery, Zacá et al cited several reports of hematoma incidence between roughly 2.5-4.3%, depending on the procedure and other mitigating factors.1 They also referenced a study by Reynolds et al in which hematomas were shown to prolong hospitalizations for up to 3.1 days at an additional cost of up to $6955.2
Hematomas occur more frequently in patients on antiplatelet therapy (single or dual, particularly clopidogrel and similar drugs) or anticoagulation (particularly IV heparin and low-molecular-weight heparin). However, recent data from a large meta-analysis showed the risk of hematoma and other complications was not increased in a large population of patients who were continued on uninterrupted oral anticoagulation.3 Other potential risk factors for hematomas include renal insufficiency and device upgrades or changeouts versus new implants as well as CRT devices. The consequences of a pocket hematoma can range from nuisance discomfort to wound issues (i.e., dehiscence), the need for reoperation, and of course, the dreaded pocket infection. In one study, 18 patients with a device infection were compared to 54 device patients without infection; researchers found that the presence of a post-op hematoma was a significant risk factor for subsequent infection (p=0.012).4 A retrospective analysis of 163 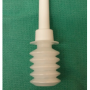 patients (45 with infection and 118 without) also found that the presence of a hematoma was associated with an increased incidence of infection (p=0.007).5
patients (45 with infection and 118 without) also found that the presence of a hematoma was associated with an increased incidence of infection (p=0.007).5
Avoidance of pocket hematoma can be aided by careful dissection of the pocket, possibly by avoidance of the single stick technique (which may lead to more local tissue disruption), use of electrocautery and/or sutures to control bleeding when it does occur, and the use of hemostatic products to minimize oozing before the pocket is closed.
A well-known adage in surgery is “dry in - dry out”, which refers to starting with a dry field and having a dry field when surgery is complete; this is an attempt to avoid subsequent complications such as hematomas and blood loss, etc. Belott and Reynolds6 discussed the so-called “wet” pocket and how to avoid and treat it, including the use of a hemostatic stitch, early pocket development to allow drying, and if necessary, the use of a drain (e.g., Jackson-Pratt).
Options to more directly decrease infection risk include the use of an antibiotic pouch or tissue pouch to help vascularize the pocket. Hemostatic options include liquid or gel thrombin to directly stimulate the clotting cascade; however, these require mixing, may leave bulk in the pocket, and do not acutely dry moisture from the pocket. SURGICEL® (Ethicon) is a biodegradable cellulose matrix that can be placed in the pocket and is used to initiate platelet aggregation and hence, hemostasis; however, it requires time for activation, is difficult to deploy over the entire pocket surface area, and has no desiccant properties. QuikClot® (Z-Medica) is a gauze impregnated with a natural mineral called kaolin that activates factor XII of the intrinsic pathway and initiates coagulation. The gauze is placed in the pocket for 3-5 minutes, pressure is applied, and then the gauze is removed; however, it also does not provide desiccant properties.
ARISTA™ AH Hemostat (Bard Davol Inc.) provides a unique option that includes hemostatic properties as well as desiccant (moisture-absorbing) properties. It is a hydrophilic, flowable, sterile, fine, dry white powder made by cross-linking purified plant starch through a proprietary process into Microporous Polysaccharide Hemospheres (MPH™). The mechanism of action is to dehydrate/concentrate blood solids such as platelets, red blood cells, and blood protein onto the beads of the product to form a gel that 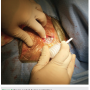 acts as a barrier to further blood loss and enhance activation of coagulation mechanisms and the formation of stable hemostatic plugs. ARISTA™ AH powder is subsequently reabsorbed by the body within 24-48 hours.
acts as a barrier to further blood loss and enhance activation of coagulation mechanisms and the formation of stable hemostatic plugs. ARISTA™ AH powder is subsequently reabsorbed by the body within 24-48 hours.
Numerous in vitro (lab studies) and in vivo (animal and human) abstracts and scientific papers discuss the benefits of this product with regard to decreasing time to hemostasis, hematomas, and blood loss. Wisman7 reported on a randomized, prospective, multicenter trial of 291 surgical patients (general, cardiac, and orthopedic) at 9 centers using ARISTA™ AH vs control. The results showed that the treatment group achieved complete hemostasis within 5 minutes (3 minutes for cardiac) in 90.3% of lesions treated with ARISTA™ AH vs 80.4% in the control group (p<0.0001). Overall, the time to hemostasis of the first treated lesion was shorter (1 minute for ARISTA™ AH vs 2 minutes for control; p=0.002). Bruckner et al8 performed a retrospective analysis on 240 patients undergoing complex cardiothoracic procedures (103 patients in the control group vs 137 patients in the ARISTA™ AH group). There was a significant decrease in hemostasis time (93.4 + 41 min in the ARISTA™ AH group vs 107.6 + 56 min in the control group; p=0.02), chest tube output at 48 hours (1594 + 949 mL in the ARISTA™ AH group vs 2112 + 1437 mL in the control group; p<0.001), and need for transfusion of blood products (2.4 + 2.5 units in the ARISTA™ AH group vs 4.0 + 5.1 units in the control group; p≤0.001) in the ARISTA™ AH group.
ARISTA™ AH comes in 1, 3, and 5 gram sizes, with the 1 gram being sufficient for device procedures. This adds roughly $120 to the cost of the case. Figure 1 shows the applicator container prior to deploying, and Figure 2 shows a device pocket during application. We recommend applying to all surfaces after placing the device in the pocket. The author (DSG) has used this product in over 3-dozen device-related procedures and has had no acute wound issues, significant hematomas, or infections.
In summary, we have found the ARISTA™ AH to be a novel product for enhancing hemostasis as well as providing pocket-drying (desiccant) properties for implantable device procedures. It is cost effective compared to other options, and has a proven track record in a variety of surgical procedures.
Disclosures: The authors have no conflicts of interest to report regarding the content herein.
References
- Zacá V, Marucci R, Parodi G, et al. Management of antithrombotic therapy in patients undergoing electrophysiology device surgery. Europace. 2015;17:840-854.
- Reynolds MR, Cohen DJ, Kugelmass AD, et al. The frequency and incremental cost of major complications among Medicare beneficiaries receiving ICDs. J Am Coll Cardiol. 2006;47:2493-2497.
- Ghanbari H, Phard WS, Al-Ameri H, et al. Meta-analysis of safety and efficacy of uninterrupted warfarin compared to heparin-based bridging therapy during the implantation of cardiac rhythm devices. Am J Cardiol. 2012;110:1482-1488.
- Raad D, Irani J, Akl EG, et al. Implantable electrophysiologic cardiac device infections: a risk factor analysis. Eur J Clin Microbiol Infect Dis. 2012;31:3015-3021.
- Martin-Casanas FV, Caballero-Estevez N, Dominguez-Rodriguez A, Abreu-Gonzalez P, Laynez-Cerdena I. Cardiac device infections is associated with pocket hematoma and diabetes mellitus: the role of the cardiovascular nurse. Int J Cardiol. 2014;171:e5-e7.
- Belott PH, Reynolds DW. Permanent pacemaker and implantable cardioverter-defibrillator implantation. In: Ellenbogen KA, Kay GN, Lau CP, Wilkoff BL, eds. Clinical Cardiac Pacing, Defibrillation, and Resynchronization Therapy. Philadelphia, PA: Elsevier-Saunders; 2011: pp. 443-515.
- Wisman CB. Arista™ AH randomized clinical study. (Reprint monograph available from Bard).
- Bruckner BA, Blau LN, Rodriguez L, et al. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J Cardiothoracic Surg. 2014;9:134.











